Korean J Urol.
2006 Dec;47(12):1354-1360. 10.4111/kju.2006.47.12.1354.
The Expression of P53 and Phosphorylation of H2AX in Germ Cells of Varicocele Rats
- Affiliations
-
- 1Department of Urology, College of Medicine, Chosun University, Gwangju, Korea. mu-hn@hanmail.net
- 2Research Center for Proteinous Materials, College of Medicine, Chosun University, Gwangju, Korea.
- 3Department of Pharmacology, College of Medicine, Chosun University, Gwangju, Korea.
- 4Department of Anatomy, College of Medicine, Chosun University, Gwangju, Korea.
- KMID: 2139783
- DOI: http://doi.org/10.4111/kju.2006.47.12.1354
Abstract
- PURPOSE
To explore the expressions of P53 and phosphorylation-H2AX in varicocele-induced rat testes.
MATERIALS AND METHODS
A total of 16 adult male Sprague-Dawley rats underwent an operation; 12 underwent an experimental varicocele and 4, as controls, were sham-operated. Groups of 4 varicocele-induced rats underwent a left orchiectomy after 2 or 3 weeks, or both orchiectomies after 4 weeks. The sham-operated rats underwent both orchiectomies after 4 weeks. Sections of both testes from each animal were studied. The changes in the expressions of P53 and phosphorylation of H2AX were determined using immunohistochemistry and western blot.
RESULTS
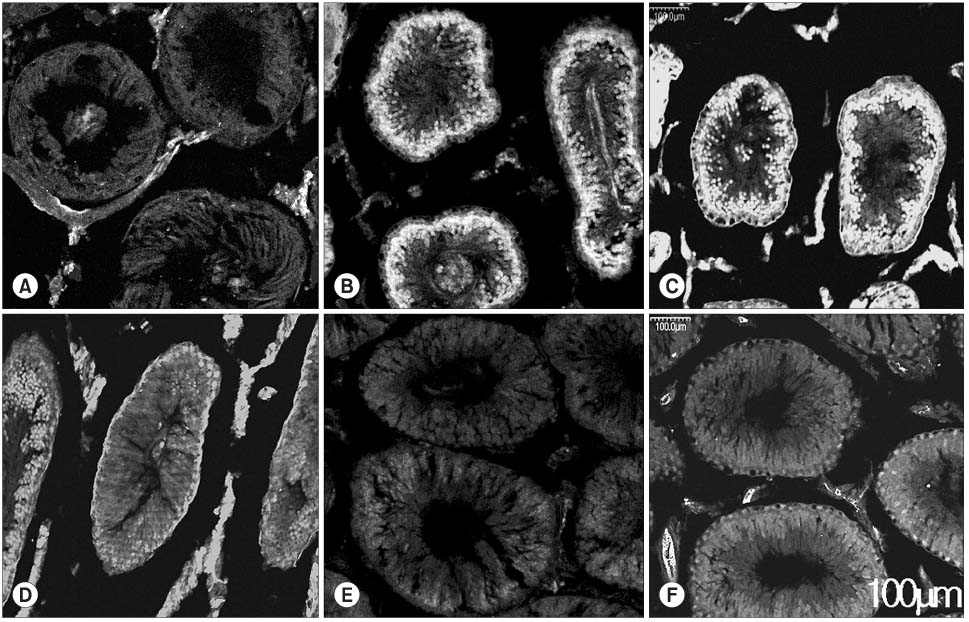
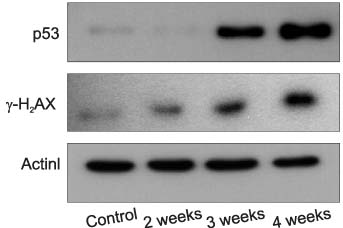
Immunohistochemical staining of the left testes in the varicocele- induced rats showed that the expressions of P53 and phosphorylation of H2AX had not begun 2 weeks postoperatively, but remarkable results were observed after 3 and 4 weeks. Both testes of the varicocele-induced rats showed the expressions of P53 and phosphorylation of H2AX after 4 weeks, with the left testes being more distinctive in immunohistochemical staining compared to the right. Western blot of the left testes in the varicocele- induced rats also showed unclear expressions of P53 and gamma-H2AX after 2 weeks. Considerable distinction was seen after 3 and 4 weeks compared to the control group.
CONCLUSIONS
Our results suggest that experimental varicocele is associated with increased sperm DNA damage. These changes may be related to abnormal spermatogenesis.
Keyword
MeSH Terms
Figure
Reference
-
1. Naughton CK, Nangia AK, Agarwal A. Pathophysiology of varicoceles in male infertility. Hum Reprod Update. 2001. 7:473–481.2. Fujisawa M, Hiramine C, Tanaka H, Okada H, Arakawa S, Kamidono S. Decrease in apoptosis of germ cells in the testes of infertile men with varicocele. World J Urol. 1999. 17:296–300.3. Agarwal A, Allamaneni SS. Sperm DNA damage assessment: a test whose time has come. Fertil Steril. 2005. 84:850–853.4. Ku J, Shim HB, Kim SW, Paick JS. The role of apoptosis in the pathogenesis of varicocele. BJU Int. 2005. 96:1092–1096.5. Saleh RA, Agarwal A, Sharma RK, Said TM, Sikka SC, Thomas AJ. Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril. 2003. 80:1431–1436.6. Agarwal A, Said TM. Oxidative stress, DNA damage and apoptosis in male infertility: a clinical approach. BJU Int. 2005. 95:503–507.7. Sinha Hikim AP, Swerdloff RS. Hormonal and genetic control of germ cell apoptosis in the testis. Rev Reprod. 1999. 4:38–47.8. Huckins C. The morphology and kinetics of spermatogonial degeneration in normal adult rats: an analysis using a simplified classification of the germinal epithelium. Anat Rec. 1978. 190:905–926.9. Fisher DE. The p53 tumor suppressor: critical regulator of life and death in cancer. Apoptosis. 2001. 6:7–15.10. Lugar K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997. 389:251–260.11. Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phophorylation on serine 139. J Biol Chem. 1998. 273:5858–5868.12. Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol. 1999. 146:905–916.13. Sedelnikova OA, Rogakou EP, Panyutin IG, Bonner WM. Quantitative detection of (125)IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat Res. 2002. 158:486–492.14. Gorelick JI, Goldstein M. Loss of infertility in men with varicocele. Fertil Steril. 1993. 59:613–616.15. Greenberg SM, Lipschultz LI, Wein AJ. Experience with 425 subfertile male patients. J Urol. 1978. 119:507–512.16. Sayfan J, Halevy A, Oland J, Nathan H. Varicocele and left renal vein compression. Fertil Steril. 1984. 41:411–417.17. Schneck FX, Bellinger MF. Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Abnormalities of the testis and scrotum and their surgical management. Campbell's urology. 1998. 8th ed. Philadelphia: Saunders;2384–2388.18. Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implication in tissue kinetics. Br J Cancer. 1972. 26:239–257.19. Barqawi A, Caruso A, Meacham RB. Experimental varicocele induces testicular germ cell apoptosis in the rat. J Urol. 2004. 171:501–503.20. Simsek F, Turkeri L, Cevik I, Bircan K, Akdas A. Role of apoptosis in testicular tissue damage caused by varicocele. Arch Esp Urol. 1998. 51:947–950.21. Kilinc F, Guvel S, Kayaselcuk F, Aygun C, Egilmez T, Ozkardes H. p53 expression and apoptosis in varicocele in the rat testis. J Urol. 2004. 172:2475–2478.22. Tanaka H, Fujisava M, Tanaka H, Okada H, Kamidono S. Apoptosis-related proteins in the testes of infertile men with varicocele. BJU Int. 2002. 89:905–909.23. Hendin BN, Kolettis PN, Sharma RK, Thomas AJ, Agarwal A. Varicocele is associated with elevated speratozoal reactive oxygen species production and diminished seminal plasma antioxidant capacity. J Urol. 1999. 161:1831–1834.24. Lane DP. Cancer. p53, guardian of the genome. Nature. 1992. 358:15–16.25. Henriksen K, Kangasniemi M, Parvinen M, Kaipia A, Hakovirta H. In vitro, follicle-stimulating hormone prevents apoptosis and stimulates deoxyribonucleic acid synthesis in the rat seminiferous epithelium in a stage-specific fashion. Endocrinology. 1996. 137:2141–2149.26. Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001. 27:247–254.27. Zweidler A. Complexity and variability of the histone complement. Life Sci Res. 1976. 4:187–196.28. Wu RS, Panusz HT, Hatch CL, Bonner WM. Histones and their modifications, CRC crit. Rev Biochem. 1986. 20:201–263.29. Chen HT, Bhandoola A, Difilippantonio MJ, Zhu J, Brown MJ, Tai X, et al. Response to RAG-mediated VDJ cleavage by NBS1 and gamma-H2AX. Science. 2000. 290:1962–1965.30. Goldberg M, Stucki M, Falck J, D'Amours D, Rahman D, Pappin D, et al. MDC1 is required for the intra-S-phase DNA damage checkpoint. Nature. 2003. 421:952–956.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of sertoli cell changes on germ cells in experimentally produced varicocele in rats
- miR-24-mediated knockdown of H2AX damages mitochondria and the insulin signaling pathway
- p53 Mutation and Expression of Rb Protein in Germ Cell Tumors
- p53-mediated Inhibitory Mechanism on HIV-1 Tat is Likely to be Associated with Tat-Phosphorylation
- Spatial and temporal expression patterns of apoptosis-related genes in rat spinal cord during normal aging