J Korean Med Assoc.
2009 Apr;52(4):405-416. 10.5124/jkma.2009.52.4.405.
Diagnostic Approaches to Patients with Thyroid Nodules
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University Medical School, Korea. drkang@chonnam.ac.kr
- KMID: 2137725
- DOI: http://doi.org/10.5124/jkma.2009.52.4.405
Abstract
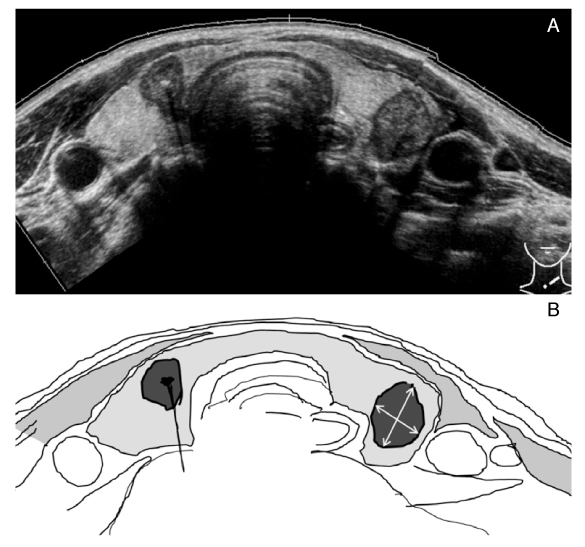
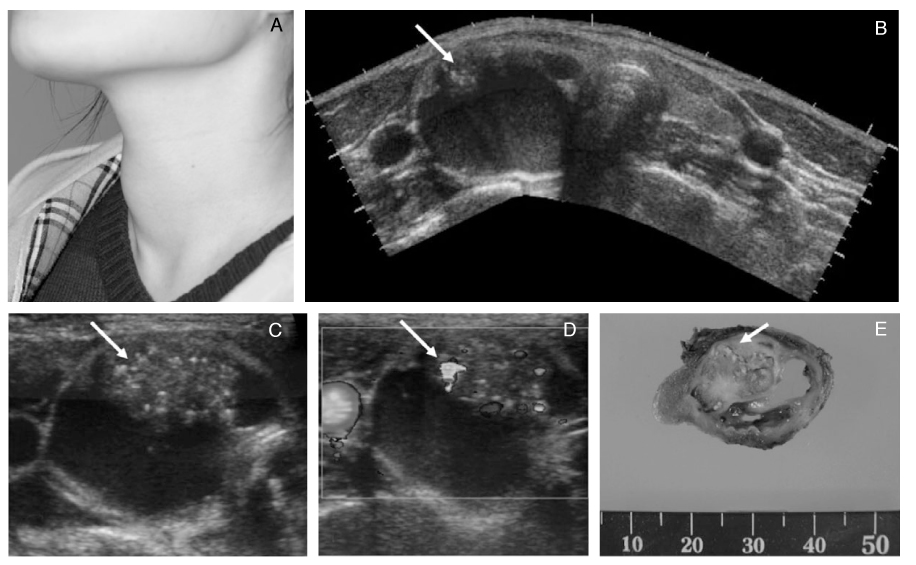
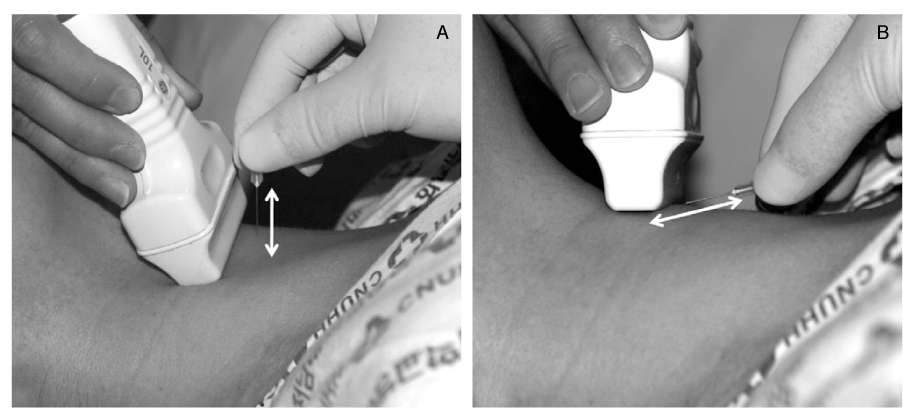
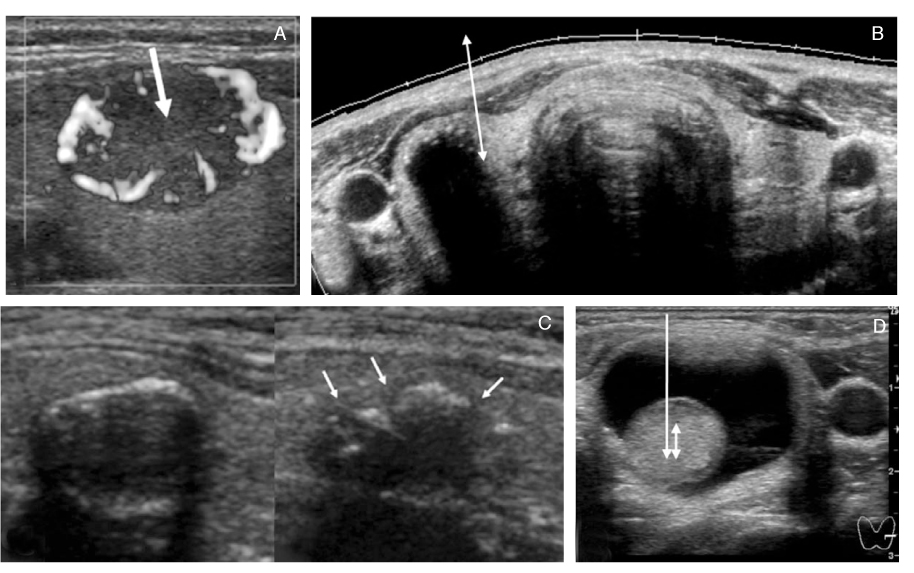
- Thyroid nodules are epidemic with the rising use of high-resolution thyroid ultrasonography for health screening. The primary aim in investigating a thyroid nodule is to exclude the possibility of malignancy, which occurs in about 5% of nodules. Initial history taking and physical examination should focus on the clinical risk factors associated with thyroid cancer. Measurement of thyroid stimulating hormone (TSH) is the only biochemical test routinely needed to exclude autonomously functioning nodules. Thyroid ultrasonography-guided fine needle aspiration biopsy (US-FNA) is the most accurate standard diagnostic test for most thyroid nodules. Ultrasonographic features of nodules associated with increased risk of thyroid cancers include hypoechogenicity, microcalcification, irregular spiculated margin, taller-than-wide, Doppler signal in the nodules, and suspicious cervical lymphadenopathies. These findings are helpful in risk stratification of the nodules and in deciding which nodule should be sampled in multinodular goiters. The success of the procedure heavily depends on the experience and expertise of the clinicians. Knowledge on basic US-FNA techniques and some tricks is very important to improve overall diagnostic yields. Practically critical issues related to US-FNA are emphasized based on several guidelines and author's experiences.
MeSH Terms
Figure
Reference
-
1. Davies L, Welch HG. Increasing Incidence of Thyroid Cancer in the United States, 1973-2002. JAMA. 2006. 295:2164–2167.
Article2. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI, Tuttle RM. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006. 16:109–142.
Article3. Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Practice & Research Clinical Endocrinology & Metabolism. 2008. 22:901–911.
Article4. Hegedus L. Clinical practice. The thyroid nodule. N Engl J Med. 2004. 351:1764–1771.5. Fukada S. Toxic multinodular goiter. Nippon Rinsho. 2006. 64:2227–2232.6. Gharib H, Papini E, Paschke R. Thyroid nodules: a review of current guidelines, practices, and prospects. Eur J Endocrinol. 2008. 159:493–505.
Article7. American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi Medical Guidelines for clinical Practice for the Diagnosis and Management of thyroid nodules. Endocrine Practice. 2006. 12:63–102.8. Frates MC, Benson CB, Charboneau JW, Cibas ES, Clark OH, Coleman BG, Cronan JJ, Doubilet PM, Evans DB, Goellner JR, Hay ID, Hertzberg BS, Intenzo CM, Jeffrey RB, Langer JE, Larsen PR, Mandel SJ, Middleton WD, Reading CC, Sherman SI, Tessler FN. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005. 237:794–800.
Article9. Kim WB, Kim TW, Kwon HS, Moon WJ, Lee JB, Choi YS, KIm SK, Kim SW, Chung KW, Baeck JH, Kim BI, Park DJ, Na DG, Choe JH, Chung JH, Jung HS, Kim JH, Nam KH, Chang HS, Chung WY, Hong SW, Hong SJ, Lee JH, Yi KH, Jo YS, Kang HC, Song M, Park JW, Yoon JH, Kang SJ, Lee KW. Management guidelines for patients with thyroid nodules and thyroid cancer. J Korean Endocr Soc. 2007. 22:157–187.
Article10. Mazzaferri EL. Management of a Solitary Thyroid Nodule. N Engl J Med. 1993. 328:553–553.
Article11. Mandel SJ. A 64-year-old woman with a thyroid nodule. JAMA. 2004. 292:2632–2642.
Article12. Castro MR, Gharib H. Continuing controversies in the management of thyroid nodules. Ann Intern Med. 2005. 142:926–931.
Article13. Brignardello E, Corrias A, Isolato G, Palestini N, Cordero di, Fagioli F, Boccuzzi G. Ultrasound Screening for Thyroid Carcinoma in Childhood Cancer Survivors: A Case Series. J Clin Endocrinol Metab. 2008. 93:4840–4843.
Article14. Bui A, Mazzaferri E. CME New Paradigms in the Diagnosis and Management of Thyroid Nodules. The Endocrinologist. 2007. 17:35.
Article15. Cases JA, Surks MI. The changing role of scintigraphy in the evaluation of thyroid nodules. Seminars in Nuclear Medicine. 2000. 30:81–87.
Article16. Hegedus L, Bonnema SJ, Bennedbaek FN. Management of simple nodular goiter: current status and future perspectives. Endocr Rev. 2003. 24:102–132.
Article17. Kang KW, Kim S-K, Kang H-S, Lee ES, Sim JS, Lee IG, Jeong S-Y, Kim SW. Prevalence and Risk of Cancer of Focal Thyroid Incidentaloma Identified by 18F -Fluorodeoxyglucose Positron Emission Tomography for Metastasis Evaluation and Cancer Screening in Healthy Subjects. J Clin Endocrinol Metab. 2003. 88:4100–4104.
Article18. Kim JM, Ryu J-S, Kim TY, Kim WB, Kwon GY, Gong G, Moon DH, Kim SC, Hong SJ, Shong YK. 18F-Fluorodeoxyglucose Positron Emission Tomography Does Not Predict Malignancy in Thyroid Nodules Cytologically Diagnosed as Follicular Neoplasm. J Clin Endocrinol Metab. 2007. 92:1630–1634.
Article19. Marqusee E, Benson CB, Frates MC, Doubilet PM, Larsen PR, Cibas ES, Mandel SJ. Usefulness of ultrasonography in the management of nodular thyroid disease. Ann Intern Med. 2000. 133:696–700.
Article20. Levine RA. Something old and something new: a brief history of thyroid ultrasound technology. Endocr Pract. 2004. 10:227–233.
Article21. Mandel SJ. Diagnostic use of ultrasonography in patients with nodular thyroid disease. Endocr Pract. 2004. 10:246–252.
Article22. Solbiati L, Osti V, Cova L, Tonolini M. Ultrasound of thyroid, parathyroid glands and neck lymph nodes. Eur Radiol. 2001. 11:2411–2424.
Article23. Ross DS. Nonpalpable Thyroid Nodules-Managing an Epidemic. J Clin Endocrinol Metab. 2002. 87:1938–1940.
Article24. Gharib H, Papini E. Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am. 2007. 36:707–735. vi.
Article25. Baskin HJ. Ultrasound-guided fine-needle aspiration biopsy of thyroid nodules and multinodular goiters. Endocr Pract. 2004. 10:242–245.
Article26. Can AS, Peker K. Comparison of palpation-versus ultrasound-guided fine-needle aspiration biopsies in the evaluation of thyroid nodules. BMC Res Notes. 2008. 1:12.
Article27. Nam-Goong Il Seong, K HY, Gong Gyungyub, Lee Ho Kyu, Hong Suck Joon, Kim Won Bae, Shong Young Kee. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clinical Endocrinology. 2004. 60:21–28.
Article28. Machens A, Holzhausen HJ, Dralle H. The prognostic value of primary tumor size in papillary and follicular thyroid carcinoma. Cancer. 2005. 103:2269–2273.
Article29. Mazzaferri EL, Sipos J. Should All Patients with Subcentimeter Thyroid Nodules Undergo Fine-Needle Aspiration Biopsy and Preoperative Neck Ultrasonography to Define the Extent of Tumor Invasion? Thyroid. 2008. 18:597–602.
Article30. Hoang JK, Lee WK, Lee M, Johnson D, Farrell S. US Features of thyroid malignancy: pearls and pitfalls. Radiographics. 2007. 27:847–860. discussion 861- 865.
Article31. Kim E-K, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, Yoo HS. New Sonographic Criteria for Recommending Fine-Needle Aspiration Biopsy of Nonpalpable Solid Nodules of the Thyroid. Am J Roentgenol. 2002. 178:687–691.
Article32. Moon W-J, Jung SL, Lee JH, Na DG, Baek J-H, Lee YH, Kim J, Kim HS, Byun JS, Lee DH. Thyroid Study Group, Korean Society of Neuro- and Head and Neck Radiology. Benign and Malignant Thyroid Nodules: US Differentiation-Multicenter Retrospective Study. Radiology. 2008. 247:762–770.
Article33. Hegedus L. Thyroid ultrasound. Endocrinol Metab Clin North Am. 2001. 30:339–360. viii-ix.
Article34. Brunese L, Romeo A, Iorio S, Napolitano G, Fucili S, Zeppa P, Vallone G, Lombardi G, Bellastella A, Biondi B, Sodano A. Thyroid B-flow twinkling sign: a new feature of papillary cancer. Eur J Endocrinol. 2008. 159:447–451.
Article35. Rago T, Santini F, Scutari M, Pinchera A, Vitti P. Elastography: New Developments in Ultrasound for Predicting Malignancy in Thyroid Nodules. J Clin Endocrinol Metab. 2007. 92:2917–2922.
Article36. Kim Won Bae, Han SM, Kim Tae Yong, Nam-Goong Il Seong, Gong Gyungyub, Lee Ho Kyu, Hong Suck Joon, Shong Young Kee. Ultrasonographic screening for detection of thyroid cancer in patients with Graves'disease. Clinical Endocrinology. 2004. 60:719–725.
Article37. Ahuja A, Chick W, King W, Metreweli C. Clinical significance of the comet-tail artifact in thyroid ultrasound. J Clin Ultrasound. 1996. 24:129–133.
Article38. Kang HC, Kim HK. Comet-tail artifact. J Korean Thyroid Assoc. 2008. 1:78–79.39. Braga M, Cavalcanti TC, Collaco LM, Graf H. Efficacy of Ultrasound-Guided Fine-Needle Aspiration Biopsy in the Diagnosis of Complex Thyroid Nodules. J Clin Endocrinol Metab. 2001. 86:4089–4091.
Article40. Poller DN, Stelow EB, Yiangou C. Thyroid FNAC cytology: can we do it better? Cytopathology. 2008. 19:4–10.
Article41. Suen KC. Fine-needle aspiration biopsy of the thyroid. CMAJ. 2002. 167:491–495.42. Alexander EK. Approach to the Patient with a Cytologically Indeterminate Thyroid Nodule. J Clin Endocrinol Metab. 2008. 93:4175–4182.
Article43. Tublin ME, Martin JA, Rollin LJ, Pealer K, Kurs-Lasky M, Ohori NP. Ultrasound-guided fine-needle aspiration versus fineneedle capillary sampling biopsy of thyroid nodules: does technique matter? J Ultrasound Med. 2007. 26:1697–1701.
Article44. Degirmenci B, Haktanir A, Albayrak R, Acar M, Sahin DA, Sahin O, Yucel A, Caliskan G. Sonographically guided fine-needle biopsy of thyroid nodules: the effects of nodule characteristics, sampling technique, and needle size on the adequacy of cytological material. Clinical Radiology. 2007. 62:798–803.
Article45. Belfiore A, La Rosa GL. Fine-needle aspiration biopsy of the thyroid. Endocrinol Metab Clin North Am. 2001. 30:361–400.
Article46. Castro MR, Gharib H. Thyroid fine-needle aspiration biopsy: progress, practice, and pitfalls. Endocr Pract. 2003. 9:128–136.
Article47. Rausch P, Nowels K, Jeffrey RB Jr. Ultrasonographically guided thyroid biopsy: a review with emphasis on technique. J Ultrasound Med. 2001. 20:79–85.
Article48. Bellantone R, Lombardi CP, Raffaelli M, Traini E, De Crea C, Rossi ED, Fadda G. Management of Cystic or Predominantly Cystic Thyroid Nodules: The Role of Ultrasound-Guided Fine-Needle Aspiration Biopsy. Thyroid. 2004. 14:43–47.
Article49. Baloch ZW, LiVolsi VA. Fine-needle aspiration of thyroid nodules: past, present, and future. Endocr Pract. 2004. 10:234–241.
Article