J Korean Soc Magn Reson Med.
2012 Dec;16(3):226-235. 10.13104/jksmrm.2012.16.3.226.
Differentitation between Primary Central Nervous System Lymphoma and Glioblastoma: Added Value of Quantitative Analysis of CT Attenuation and Apparent Diffusion Coefficient
- Affiliations
-
- 1Department of Radiology, Konkuk University School of Medicine, Seoul, Korea. mdmoonwj@naver.com
- 2Department of Radiology, Konkuk University Medical Center, Seoul, Korea.
- 3Department of Radiology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2099858
- DOI: http://doi.org/10.13104/jksmrm.2012.16.3.226
Abstract
- PURPOSE
Purpose of this study was to determine if quantitative measures of CT attenuation and ADC values in combination with conventional imaging features can differentiate primary central nervous system lymphoma (PCNSL) and glioblastoma (GBM).
MATERIALS AND METHODS
Twenty-six patients with histologically-proven GBM (14 men and 12 women; median age, 50 years; age range, 22 - 73 years) and 14 patients with PCNSL (11 men and 3 women; median age, 61 years; age range, 41 - 74 years) were enrolled. Maximum CT attenuation, minimum ADC, and lesion to normal parenchyma minimum ADC ratios were measured in solid tumor regions. Conventional imaging features were evaluated for the following: ill-defined margin, homogeneous enhancement pattern, degree of necrosis, extent of tumor involvement and multiplicity. The Mann-Whitney test was used to compare maximum CT attenuation and minimum ADC values for PCNSL and GBM. Fisher's exact test was used to evaluate relationships between pathologic diagnoses and imaging features.
RESULTS
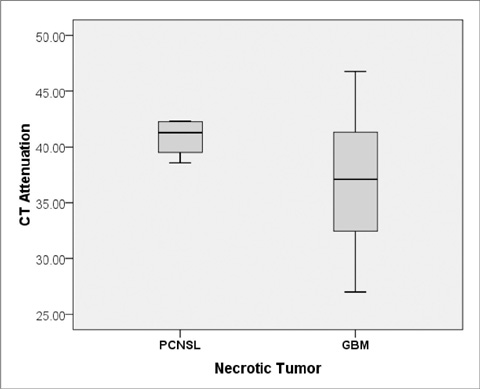
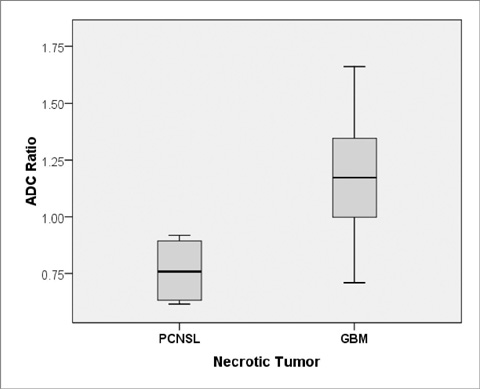
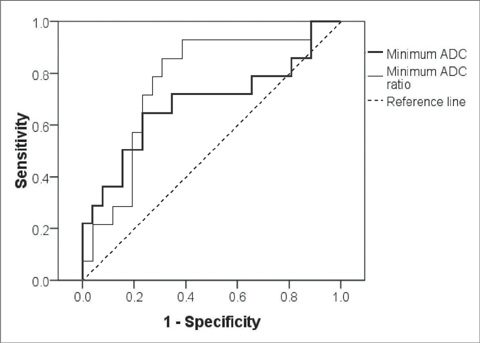
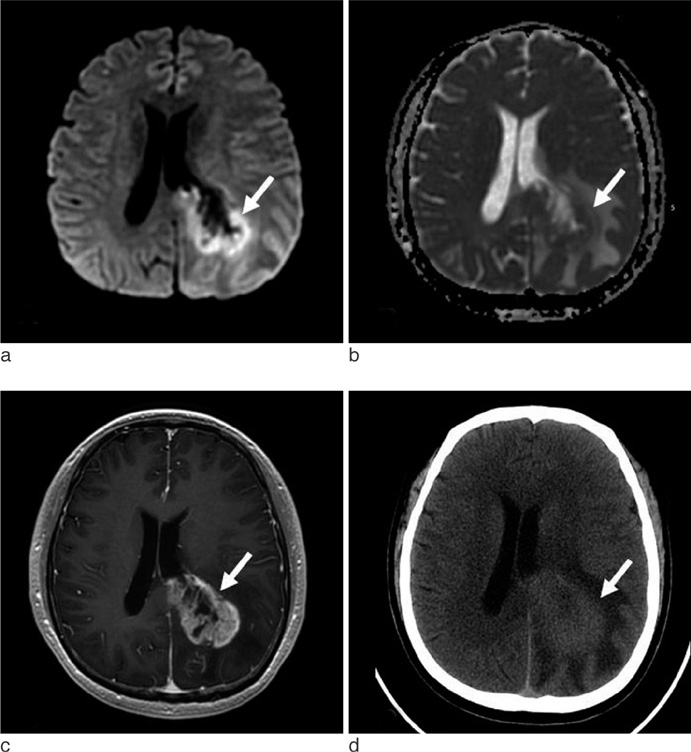
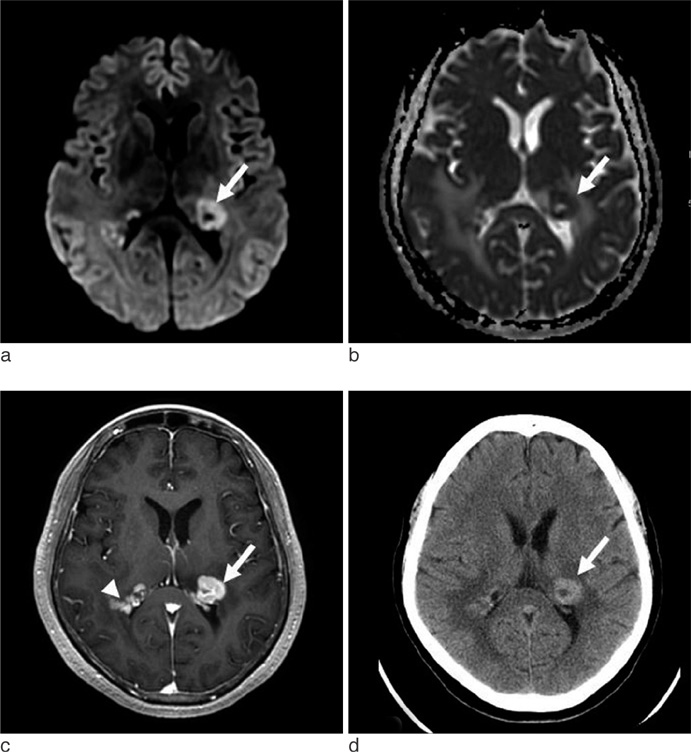
The CT attenuations were similar for PCNSL and GBM (37.84 +/- 6.90 HU versus 37.00 +/- 5.54 HU, p = 0.68), but minimum ADC and minimum ADC ratio were significant lower in PCNSL than in GBM (595.01 +/- 228.28 10(-6) mm2/s versus 736.52 +/- 162.05 10(-6) mm2/s; p = 0.028, 0.87 +/- 0.26 versus 1.14 +/- 0.29; p = 0.007). PCNSL showed greater homogeneous enhancement and smaller necrotic areas than GBM (p = 0.003 and p < 0.001, respectively) and was more likely to have multiple tumors than GBM (p = 0.039). When necrotic PCNSL (n = 4) and necrotic GBM (n = 24) were compared, minimum ADC and minimum ADC ratios were also significantly lower in PCNSL, but CT attenuation were not.
CONCLUSION
Although CT attenuation does not provide valuable information, minimum ADC and minimum ADC ratio and some imaging features can aid the differentiation of PCNSL and GBM.
Keyword
Figure
Reference
-
1. Haldorsen IS, Krossnes BK, Aarseth JH, et al. Increasing incidence and continued dismal outcome of primary central nervous system lymphoma in Norway 1989-2003 : time trends in a 15-year national survey. Cancer. 2007. 110:1803–1814.2. Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol. 2011. 32:984–992.3. Shah GD, DeAngelis LM. Treatment of primary central nervous system lymphoma. Hematol Oncol Clin North Am. 2005. 19:611–627.4. O'Brien PC, Roos DE, Pratt G, et al. Combined-modality therapy for primary central nervous system lymphoma: long-term data from a Phase II multicenter study (Trans-Tasman Radiation Oncology Group). Int J Radiat Oncol Biol Phys. 2006. 64:408–413.5. Elder JB, Chen TC. Surgical interventions for primary central nervous system lymphoma. Neurosurg Focus. 2006. 21:E13.6. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005. 352:987–996.7. Pichlmeier U, Bink A, Schackert G, Stummer W. Resection and survival in glioblastoma multiforme: an RTOG recursive partitioning analysis of ALA study patients. Neuro Oncol. 2008. 10:1025–1034.8. Stummer W, van den Bent MJ, Westphal M. Cytoreductive surgery of glioblastoma as the key to successful adjuvant therapies: new arguments in an old discussion. Acta Neurochir (Wien). 2011. 153:1211–1121.9. Koeller KK, Smirniotopoulos JG, Jones RV. Primary central nervous system lymphoma: radiologic-pathologic correlation. Radiographics. 1997. 17:1497–1526.10. Coulon A, Lafitte F, Hoang-Xuan K, et al. Radiographic findings in 37 cases of primary CNS lymphoma in immunocompetent patients. Eur Radiol. 2002. 12:329–340.11. Go JL, Lee SC, Kim PE. Imaging of primary central nervous system lymphoma. Neurosurg Focus. 2006. 21:E4.12. Hartmann M, Heiland S, Harting I, et al. Distinguishing of primary cerebral lymphoma from high-grade glioma with perfusion-weighted magnetic resonance imaging. Neurosci Lett. 2003. 338:119–122.13. Cianfoni A, Colosimo C, Basile M, Wintermark M, Bonomo L. Brain perfusion CT: principles, technique and clinical applications. Radiol Med. 2007. 112:1225–1243.14. Zacharia TT, Law M, Naidich TP, Leeds NE. Central nervous system lymphoma characterization by diffusion-weighted imaging and MR spectroscopy. J Neuroimaging. 2008. 18:411–417.15. Stadnik TW, Chaskis C, Michotte A, et al. Diffusion-weighted MR imaging of intracerebral masses: comparison with conventional MR imaging and histologic findings. AJNR Am J Neuroradiol. 2001. 22:969–976.16. Toh CH, Castillo M, Wong AM, et al. Primary cerebral lymphoma and glioblastoma multiforme: differences in diffusion characteristics evaluated with diffusion tensor imaging. AJNR Am J Neuroradiol. 2008. 29:471–475.17. Moon WJ, Choi JW, Roh HG, Lim SD, Koh YC. Imaging parameters of high grade gliomas in relation to the MGMT promoter methylation status: the CT, diffusion tensor imaging, and perfusion MR imaging. Neuroradiology. 2012. 54:555–563.18. Kim DS, Na DG, Kim KH, et al. Distinguishing tumefactive demyelinating lesions from glioma or central nervous system lymphoma: added value of unenhanced CT compared with conventional contrast-enhanced MR imaging. Radiology. 2009. 251:467–475.19. Parizel PM, Makkat S, Van Miert E, Van Goethem JW, van den Hauwe L, De Schepper AM. Intracranial hemorrhage: principles of CT and MRI interpretation. Eur Radiol. 2001. 11:1770–1783.20. Erdag N, Bhorade RM, Alberico RA, Yousuf N, Patel MR. Primary lymphoma of the central nervous system: typical and atypical CT and MR imaging appearances. AJR Am J Roentgenol. 2001. 176:1319–1326.21. Guo AC, Cummings TJ, Dash RC, Provenzale JM. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology. 2002. 224:177–183.22. Horger M, Fenchel M, Nagele T, et al. Water diffusivity: comparison of primary CNS lymphoma and astrocytic tumor infiltrating the corpus callosum. AJR Am J Roentgenol. 2009. 193:1384–1387.23. Sasaki M, Yamada K, Watanabe Y, et al. Variability in absolute apparent diffusion coefficient values across different platforms may be substantial: a multivendor, multi-institutional comparison study. Radiology. 2008. 249:624–630.24. Haldorsen IS, Krakenes J, Krossnes BK, Mella O, Espeland A. CT and MR imaging features of primary central nervous system lymphoma in Norway, 1989-2003. AJNR Am J Neuroradiol. 2009. 30:744–751.25. Kuker W, Nagele T, Korfel A, et al. Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J Neurooncol. 2005. 72:169–177.26. Hayakawa T, Takakura K, Abe H, et al. Primary central nervous system lymphoma in Japan--a retrospective, co-operative study by CNS-Lymphoma Study Group in Japan. J Neurooncol. 1994. 19:197–215.27. Slone HW, Blake JJ, Shah R, Guttikonda S, Bourekas EC. CT and MRI findings of intracranial lymphoma. AJR Am J Roentgenol. 2005. 184:1679–1685.28. Barami K, Sloan AE, Rojiani A, Schell MJ, Staller A, Brem S. Relationship of gliomas to the ventricular walls. J Clin Neurosci. 2009. 16:195–201.29. Lim DA, Cha S, Mayo MC, et al. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol. 2007. 9:424–429.30. Buhring U, Herrlinger U, Krings T, Thiex R, Weller M, Kuker W. MRI features of primary central nervous system lymphomas at presentation. Neurology. 2001. 57:393–396.31. Fine HA, Mayer RJ. Primary central nervous system lymphoma. Ann Intern Med. 1993. 119:1093–1104.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Primary Central Nervous System Lymphoma in Organ Recipient
- Primary Central Nervous System Lymphoma Mimicking Behcet's Disease
- Apparent diffusion coefficient as a valuable quantitative parameter for predicting clinical outcomes in patients with newly diagnosed primary CNS lymphoma
- 1H Magnetic Resonance Spectroscopy of Primary Central Nervous System Lymphoma
- Experience of Childhood Non-Hodgkin Lymphoma with Central Nervous System Involvement at Diagnosis