Korean Circ J.
2012 Jul;42(7):479-486. 10.4070/kcj.2012.42.7.479.
Probing Regulatory Proteins for Vascular Contraction by Deoxyribonucleic Acid Microarray
- Affiliations
-
- 1Department of Pharmacology, Kyungpook National University School of Medicine, Daegu, Korea. inkim@knu.ac.kr
- 2Cardiovascular Research Institute, Kyungpook National University School of Medicine, Daegu, Korea.
- KMID: 2094120
- DOI: http://doi.org/10.4070/kcj.2012.42.7.479
Abstract
- BACKGROUND AND OBJECTIVES
The heat-shock response modulates contractility of vascular smooth muscles. With complementary deoxyribonucleic acid microarray, we tried to identify the novel genes that are involved in the regulation of vascular contraction after heat shock.
MATERIALS AND METHODS
Human radial artery strips were mounted in organ baths, exposed at 42degrees C for 45 minutes, and returned to equilibrate at 37degrees C. This study examined gene expression profile associated with heat-shock response in radial arteries of patients with hyperlipidemia by using a microarray that contained 5763 human cDNA. The results of microarray hybridization experiments from the radial arteries of 4 different subjects were analyzed and classified by the cluster program.
RESULTS
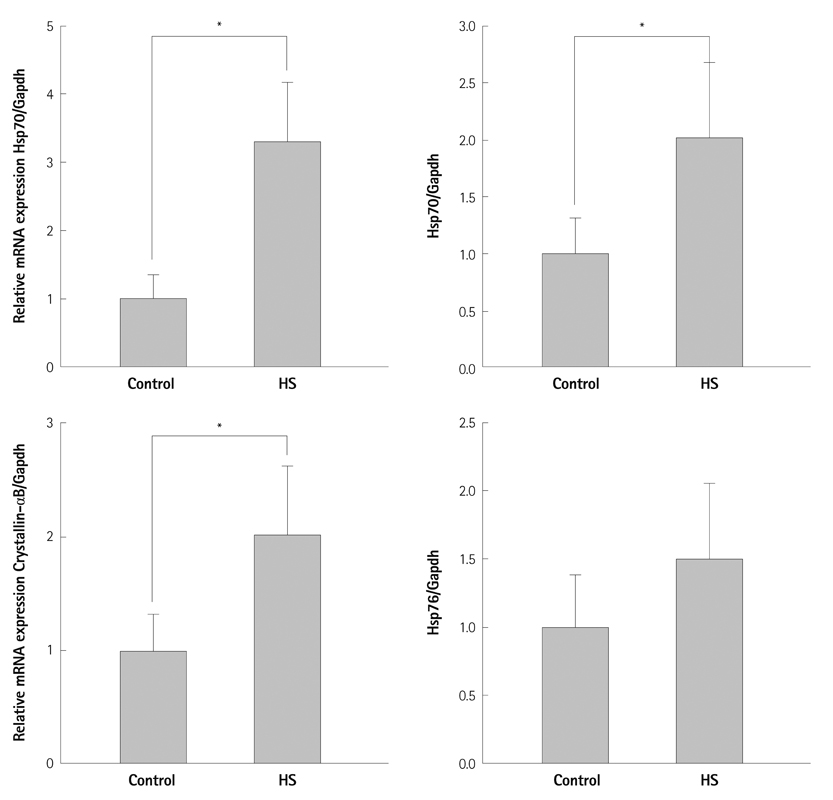
Among these differentially-expressed genes, Hsp70, Hsp10, alphaB-crystallin, and Hsp60 were significantly increased by the heat shock response. Of non-HSP genes, 15 genes increased, while 22 genes decreased. Among these 37 genes, alphaB-crystallin (CRYAB) (up 1.92-fold), myosin, light polypeptide kinase transcript variant 8, 6 (up 1.70-fold, up 1.68-fold), catenin (cadherin-associated protein, alpha-like 1) (down-0.57 fold) and tropomyosin 3 (down 0.68-fold) were thought to be related with the contraction. Real-time quantitative polymerase chain reaction showed that Hsp70, Hsp10 and alphaB-crystallin were significantly increased.
CONCLUSION
Gene expression profile by heat shock provides information about genes implicated in augmentation of vascular contraction after heat shock.
Keyword
MeSH Terms
Figure
Reference
-
1. Snoeckx LH, Cornelussen RN, Van Nieuwenhoven FA, Reneman RS, Van Der Vusse GJ. Heat shock proteins and cardiovascular pathophysiology. Physiol Rev. 2001. 81:1461–1497.2. Park HW, Kown TG, Kim KY, Bae JH. Diabetes, insulin resistance and atherosclerosis surrogates in patients with coronary atherosclerosis. Korean Circ J. 2010. 40:62–67.3. Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988. 22:631–677.4. Xu Q. Role of heat shock proteins in atherosclerosis. Arterioscler Thromb Vasc Biol. 2002. 22:1547–1559.5. An HS, Bae EJ, Kim GB, et al. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J. 2010. 40:131–136.6. Seo J, Kim M, Kim J. Identification of novel genes differentially expressed in PMA-induced HL-60 cells using cDNA microarrays. Mol Cells. 2000. 10:733–739.7. Luo L, Salunga RC, Guo H, et al. Gene expression profiles of laser-captured adjacent neuronal subtypes. Nat Med. 1999. 5:117–122.8. Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998. 95:14863–14868.9. Xu Q, Li DG, Holbrook NJ, Udelsman R. Acute hypertension induces heat-shock protein 70 gene expression in rat aorta. Circulation. 1995. 92:1223–1229.10. Lee G, Oh Y, Lee J. Upregulation of heat shock proteins in the kidney in hypertension. Korean J Physiol Pharmacol. 2004. 8:147–151.11. Meyer AS, Gillespie JR, Walther D, Millet IS, Doniach S, Frydman J. Closing the folding chamber of the eukaryotic chaperonin requires the transition state of ATP hydrolysis. Cell. 2003. 113:369–381.12. Morton H. Early pregnancy factor: an extracellular chaperonin 10 homologue. Immunol Cell Biol. 1998. 76:483–496.13. Cavanagh AC. Identification of early pregnancy factor as chaperonin 10: implications for understanding its role. Rev Reprod. 1996. 1:28–32.14. Johnson GB, Brunn GJ, Platt JL. Activation of mammalian Toll-like receptors by endogenous agonists. Crit Rev Immunol. 2003. 23:15–44.15. Wallin RP, Lundqvist A, Moré SH, von Bonin A, Kiessling R, Ljunggren HG. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002. 23:130–135.16. Kim IK, Park TG, Kim YH, Cho JW, Kang BS, Kim CY. Heat-shock response is associated with enhanced contractility of vascular smooth muscle in isolated rat aorta. Naunyn Schmiedebergs Arch Pharmacol. 2004. 369:402–407.17. Groenen PJ, Merck KB, de Jong WW, Bloemendal H. Structure and modifications of the junior chaperone alpha-crystallin: from lens transparency to molecular pathology. Eur J Biochem. 1994. 225:1–19.18. Datta SA, Rao CM. Differential temperature-dependent chaperone-like activity of alphaA- and alphaB-crystallin homoaggregates. J Biol Chem. 1999. 274:34773–34778.19. Geeves MA, Fedorov R, Manstein DJ. Molecular mechanism of actomyosin-based motility. Cell Mol Life Sci. 2005. 62:1462–1477.20. Barral JM, Hutagalung AH, Brinker A, Hartl FU, Epstein HF. Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science. 2002. 295:669–671.21. Srikakulam R, Winkelmann DA. Chaperone-mediated folding and assembly of myosin in striated muscle. J Cell Sci. 2004. 117(Pt 4):641–652.22. Shirinsky VP, Vorotnikov AV, Birukov KG, et al. A kinase-related protein stabilizes unphosphorylated smooth muscle myosin minifilaments in the presence of ATP. J Biol Chem. 1993. 268:16578–16583.23. Silver DL, Vorotnikov AV, Watterson DM, Shirinsky VP, Sellers JR. Sites of interaction between kinase-related protein and smooth muscle myosin. J Biol Chem. 1997. 272:25353–25359.24. Lim KA, Kim KC, Cho MS, Lee BE, Kim HS, Hong YM. Gene expression of endothelin-1 and endothelin receptor A on monocrotaline-induced pulmonary hypertension in rats after bosentan treatment. Korean Circ J. 2010. 40:459–464.25. Lees-Miller JP, Helfman DM. The molecular basis for tropomyosin isoform diversity. Bioessays. 1991. 13:429–437.26. Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000. 80:853–924.27. Cipolla MJ, Gokina NI, Osol G. Pressure-induced actin polymerization in vascular smooth muscle as a mechanism underlying myogenic behavior. FASEB J. 2002. 16:72–76.28. Tang DD, Tan J. Downregulation of profilin with antisense oligodeoxynucleotides inhibits force development during stimulation of smooth muscle. Am J Physiol Heart Circ Physiol. 2003. 285:H1528–H1536.29. Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005. 123:903–915.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tyrosine phosphorylation of paxillin may be involved in vascular smooth muscle contraction
- High fat diet confers vascular hyper-contractility against angiotensin II through upregulation of MLCK and CPI-17
- Differential effects of saturated and unsaturated fatty acids on vascular reactivity in isolated mesenteric and femoral arteries of rats
- Possible Roles of UL112-113 Proteins in Human Cytomegalovirus DNA Replication
- Basic Understanding of Iron Metabolism