Korean J Hematol.
2005 Dec;40(4):211-218. 10.5045/kjh.2005.40.4.211.
siRNA Targeting Vascular Endothelial Growth Factor and Recombinant Human Prothrombin Kringle 2 Inhibits Leukemia-induced Angiogenesis
- Affiliations
-
- 1Department of Biochemistry, College of Science, Yonsei University, Seoul, Korea. kimss518@yonsei.ac.kr
- KMID: 2083458
- DOI: http://doi.org/10.5045/kjh.2005.40.4.211
Abstract
- BACKGROUND
Vascular endothelial growth factor (VEGF) plays a role in the development of cancer and the progression of liquid tumors such as chronic lymphatic leukemia, non-Hodgkin lymphomas, and multiple myeloma. VEGF also triggers endothelial cells to secrete hematopoietic growth factors such as interleukin-6 (IL-6); this in turn promotes further leukemia growth, thereby contributing to a paracrine loop between the leukemia and the endothelial cells.
METHODS
We transfected a small interfering RNA (siRNA) targeting VEGF into K562 cells in order to investigate the role of VEGF in the development of leukemic cancer. After the conditioned media (CM) of the K562 was cells added to the human umbilical endothelial cell (HUVEC) culture media, we compared the proliferation and tube formation of the HUVECs. Recombinant human prothrombin kringle2 (K2), which is a known angiogenic inhibitor, was also treated onto the HUVECs, and we then examined the level of IL-6 to determine the paracrine interaction between the leukemic and endothelial cells.
RESULTS
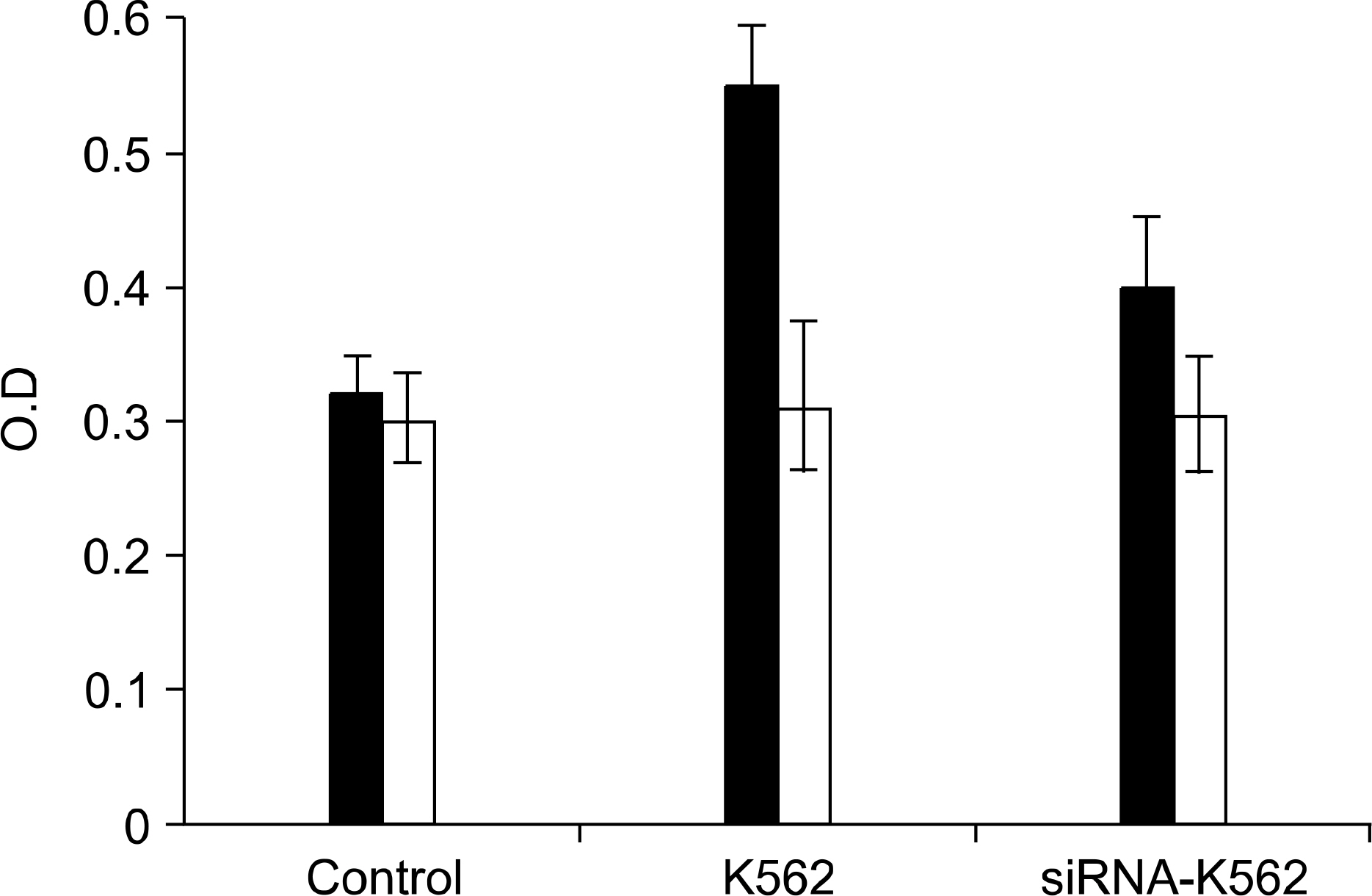
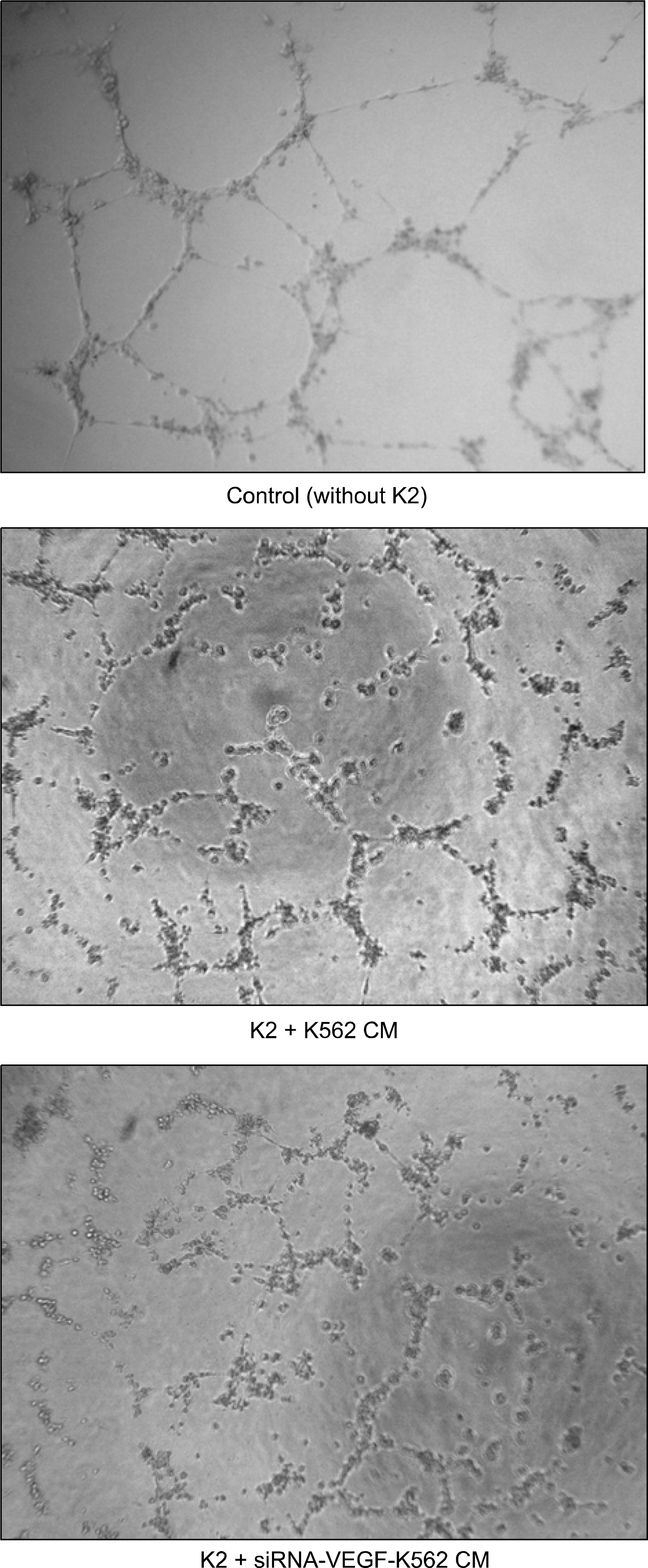
RT-PCR and western blot analysis demonstrated that the siRNA efficiently down regulated the expression of VEGF in the K562 cells. When the CM of the K562 cells was added to the HUVEC culture, the proliferation of the HUVECs was stimulated. The proliferation of the HUVEC induced by the CM from the siRNA-VEGF K562 cells was diminished, compared with that of the vector control K562 cells. K2 reduced not only the proliferation of the HUVECs, but also the secretion of IL-6 by the HUVEC.
CONCLUSION
The siRNA strategy is an alternative tool for inhibiting leukemia-induced angiogenesis. A combinated therapy with angiogenesis inhibitor K2 increases the efficiency. K2 modulates the production of IL-6, which may affect the paracrine interaction between leukemia and endothelial cells.
MeSH Terms
-
Blotting, Western
Culture Media
Culture Media, Conditioned
Endothelial Cells
Humans*
Intercellular Signaling Peptides and Proteins
Interleukin-6
K562 Cells
Kringles*
Leukemia
Lymphoma, Non-Hodgkin
Multiple Myeloma
Prothrombin*
RNA, Small Interfering*
Vascular Endothelial Growth Factor A*
Culture Media
Culture Media, Conditioned
Intercellular Signaling Peptides and Proteins
Interleukin-6
Prothrombin
RNA, Small Interfering
Vascular Endothelial Growth Factor A
Figure
Reference
-
1). Senger DR, Van de Water L, Brown LF, et al. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 1993; 12:303–24.
Article2). Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999; 77:527–43.
Article3). Chen H, Treweeke AT, West DC, et al. In vitro and in vivo production of vascular endothelial growth factor by chronic lymphatic leukemia cells. Blood. 2000; 96:3181–7.4). Schuch G, Machluf M, Bartsch G Jr, et al. In vivo administration of vascular endothelial growth factor (VEGF) and its antagonist, soluble neuropilin-1, predicts a role of VEGF in the progression of acute myeloid leukemia in vivo. Blood. 2002; 100:4622–8.
Article5). Zhang HT, Craft P, Scott PA, et al. Enhancement of tumor growth and vascular density by transfection of vascular endothelial cell growth factor into MCF-7 human breast carcinoma cells. J Natl Cancer Inst. 1995; 87:213–9.
Article6). Ciardiello F, Bianco R, Damiano V, et al. Antian-giogenic and antitumor activity of anti-epidermal growth factor receptor C225 monoclonal antibody in combination with vascular endothelial growth factor antisense oligonucleotide in human GEO colon cancer cells. Clin Cancer Res. 2000; 6:3739–47.7). Nakashima T, Hudson JM, Clayman GL. Antisense inhibition of vascular endothelial growth factor in human head and neck squamous cell carcinoma. Head Neck. 2000; 22:483–8.
Article8). Im SA, Gomez-Manzano C, Fueyo J, et al. Antian-giogenesis treatment for gliomas: transfer of antisense-vascular endothelial growth factor inhibits tumor growth in vivo. Cancer Res. 1999; 59:895–900.9). Dankdar B, Padro T, Leo R, et al. Vascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myeloma. Blood. 2000; 95:2630–6.10). Rhim TY, Kim E, Park CS, Kim SS. Expression, purification and characterization of prothrombin kringle 2. J Biochem Mol Biol. 1999; 32:147–53.11). Kim BJ, Koo SY, Kim SS. A peptide derived from human prothrombin fragment 2 inhibits prothrombinase and angiogenesis. Thromb Res. 2002; 106:81–7.
Article12). Perez-Atayde AR, Sallan SE, Tedrow U, Connors S, Allred E, Folkman J. Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am J Pathol. 1997; 150:815–21.13). Hussong JW, Rodgers GM, Shami PJ. Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood. 2000; 95:309–13.
Article14). Aguayo A, Kantarjian H, Mansbouri T, et al. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood. 2000; 96:2240–5.
Article15). Aguayo A, Estey E, Kantarjian H, et al. Cellular vascular endothelial growth factor is a predictor of outcome in patients with acute myeloid leukemia. Blood. 1999; 94:3717–21.
Article16). Fusetti L, Pruneri G, Gobbi A, et al. Human myeloid and lymphoid malignancies in the non-obese diabe-tic/severe combined immunodeficiency mouse model: frequency of apoptotic cells in solid tumors and efficiency and speed of engraftment correlate with vascular endothelial growth factor production. Cancer Res. 2000; 60:2527–34.17). Salven P, Orpana A, Teerenhovi L, Joensuu H. Simultaneous elevation in the serum concentrations of the angiogenic growth factors VEGF and bFGF is an independent predictor of poor prognosis in non-Hodgkin lymphoma: a single institution study of 200 patients. Blood. 2000; 96:3712–8.18). Molica S, Vitelli G, Levato D, Gandolfo GM, Liso V. Increased serum levels of vascular endothelial growth factor predict risk of progression in early B-cell chronic lymphocytic leukaemia. Br J Haematol. 1999; 107:605–10.
Article19). He R, Liu B, Yang C, Yang RC, Tobelem G, Han ZC. Inhibition of K562 leukemia angiogenesis and growth by expression of antisense vascular endothelial growth factor (VEGF) sequence. Cancer Gene Ther. 2003; 10:879–86.
Article20). Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001; 411:494–8.
Article21). Takei Y, Kadomatsu K, Yuzawa Y, Matsuo S, Muramatsu T. A small interfering RNA targeting vascular endothelial growth factor as cancer therapeutics. Cancer Res. 2004; 64:3365–70.
Article22). Dankbar B, Padro T, Leo R, et al. Vascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myeloma. Blood. 2000; 95:2630–6.
Article23). Hirano T, Akira S, Taga T, Kishimoto T. Biological and clinical aspects of interleukin 6. Immunol Today. 1990; 11:443–9.
Article24). Bataille R, Jourdan M, Zhang XG, Klein B. Serum levels of interleukin 6, a potent myeloma cell growth factor, as a reflect of disease severity in plasma cell dyscrasias. J Clin Invest. 1989; 84:2008–11.
Article25). Tsukamoto T, Kumamoto Y, Miyao N, Masumori N, Takahashi A, Yanase M. Interleukin-6 in renal cell carcinoma. J Urol. 1992; 148:1778–81.
Article26). Berek JS, Chung C, Kaldi K, Watson JM, Knox RM, Martinez-Maza O. Serum interleukin-6 levels correlate with disease status in patients with epithelial ovarian cancer. Am J Obstet Gynecol. 1991; 164:1038–42.
Article27). Wu CW, Wang SR, Chao MF, et al. Serum interleukin-6 levels reflect disease status of gastric cancer. Am J Gastroenterol. 1996; 91:1417–22.28). Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996; 271:736–41.
Article29). Garber K. Angiogenesis inhibitors suffer new setback. Nat Biotechnol. 2002; 20:1067–8.
Article30). O'Reilly MS. Targeting multiple biological pathways as a strategy to improve the treatment of cancer. Clin Cancer Res. 2002; 8:3309–10.31). Kim KW, Kim HJ, Park SY. The relationship between clinical drug sensitivity and expression of drug resistance genes in patients with acute myelogenous leukemia. Korean J Hematol. 2001; 36:115–22.32). Li M, Ye C, Feng C, et al. Enhanced antiangiogenic therapy of squamous cell carcinoma by combined endostatin and epidermal growth factor receptor-antisense therapy. Clin Cancer Res. 2002; 8:3570–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Zerumbone, Sesquiterpene Photochemical from Ginger, Inhibits Angiogenesis
- Thrombospondin-1 and Inhibition of Tumor Growth
- Role of Angiogenic Factors during the Hepatocarcinogenesis
- The inhibitory effects of recombinant plasminogen kringle 1-3 on the neovascularization of rabbit cornea induced by angiogenin, bFGF, and VEGF
- Pivotal role of vascular endothelial growth factor pathway in tumor angiogenesis