Korean J Pain.
2013 Oct;26(4):356-360. 10.3344/kjp.2013.26.4.356.
The Effect of Urinary Trypsin Inhibitor Against Neuropathic Pain in Rat Models
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chosun University School of Medicine, Gwangju, Korea. kjlim@chosun.ac.kr
- 2Department of Anesthesiology and Pain Medicine, Medical School, Chonnam National University, Gwangju, Korea.
- KMID: 2074043
- DOI: http://doi.org/10.3344/kjp.2013.26.4.356
Abstract
- BACKGROUND
Nerve injury sometimes leads to chronic neuropathic pain associated with neuroinflammation in the nervous system. In the case of chronic neuropathic pain, the inflammatory and algesic mediators become predominant and result in pain hypersensitivity following nervous system damage. It is well known that urinary trypsin inhibitor (ulinastatin, UTI) has an anti-inflammatory activity. Recently, the neuroprotective action of UTI on the nervous system after ischemic injury has been reported. Thus, we evaluated the neuroprotective effect of ulinastatin in a rat model of neuropathic pain.
METHODS
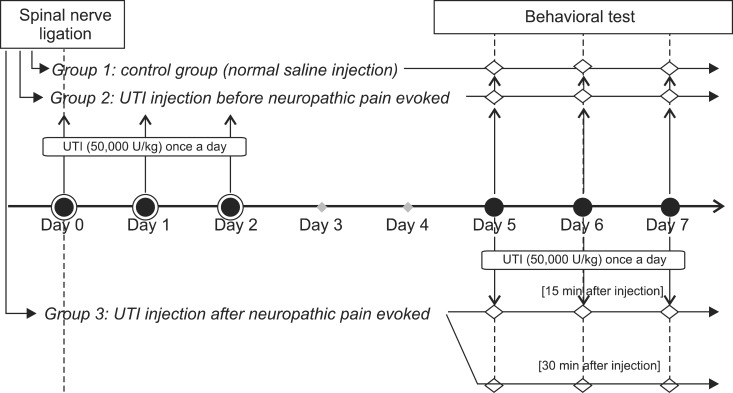
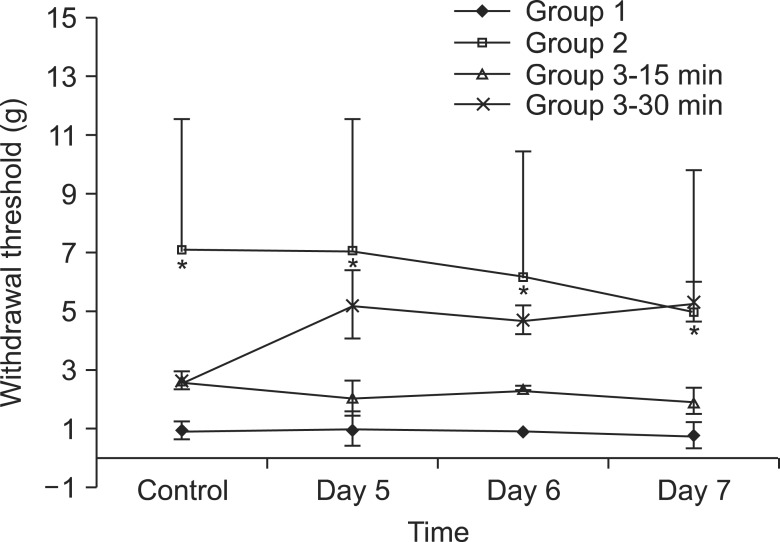
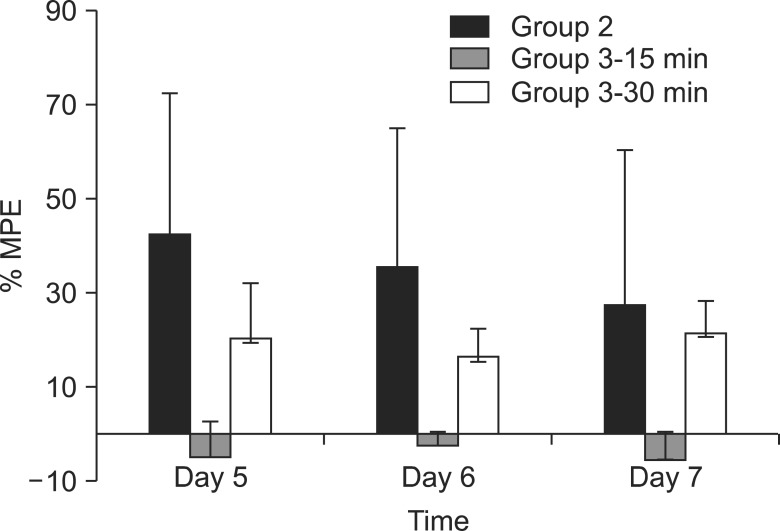
Neuropathic pain was induced with L5 spinal nerve ligation (SNL) in male Sprague-Dawley rats weighing 100-120 g. The rats were divided into 3 groups, with n = 8 in each group. The rats in the control group (group 1) were administered normal saline and those in group 2 were administered UTI (50,000 U/kg) intravenously through the tail vein for 3 days from the day of SNL. Rats in group 3 were administered UTI (50,000 U/kg) intravenously from the 5th day after SNL. The paw withdrawal threshold was measured using the von Frey test for 3 days starting from the 5th day after SNL.
RESULTS
The paw withdrawal thresholds were significantly increased in the rats of group 2 compared to the other groups (P < 0.05).
CONCLUSIONS
Ulinastatin, which was administered for 3 days after SNL, increased the paw withdrawal threshold and it could have a neuroprotective effect in the rat model of neuropathic pain.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Gabexate mesilate ameliorates the neuropathic pain in a rat model by inhibition of proinflammatory cytokines and nitric oxide pathway
via suppression of nuclear factor-κB
Seon Hee Oh, Hyun Young Lee, Young Joon Ki, Sang Hun Kim, Kyung Joon Lim, Ki Tae Jung
Korean J Pain. 2020;33(1):30-39. doi: 10.3344/kjp.2020.33.1.30.Olanzapine Attenuates Mechanical Allodynia in a Rat Model of Partial Sciatic Nerve Ligation
Taeko Fukuda, Soichiro Yamashita, Setsuji Hisano, Makoto Tanaka
Korean J Pain. 2015;28(3):185-192. doi: 10.3344/kjp.2015.28.3.185.Autophagy: Can It be a New Experimental Research Method of Neuropathic Pain?
Ki Tae Jung, Kyung Joon Lim
Korean J Pain. 2015;28(4):229-230. doi: 10.3344/kjp.2015.28.4.229.
Reference
-
1. Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010; 229:26–50. PMID: 20870295.
Article2. Üçeyler N, Sommer C. Cytokine regulation in animal models of neuropathic pain and in human diseases. Neurosci Lett. 2008; 437:194–198. PMID: 18403115.
Article3. Thacker MA, Clark AK, Marchand F, McMahon SB. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg. 2007; 105:838–847. PMID: 17717248.
Article4. Park KH, Lee KH, Kim H, Hwang SO. The anti-inflammatory effects of ulinastatin in trauma patients with hemorrhagic shock. J Korean Med Sci. 2010; 25:128–134. PMID: 20052358.
Article5. Aosasa S, Ono S, Mochizuki H, Tsujimoto H, Ueno C, Matsumoto A. Mechanism of the inhibitory effect of protease inhibitor on tumor necrosis factor alpha production of monocytes. Shock. 2001; 15:101–105. PMID: 11220636.
Article6. Park JH, Kwak SH, Jeong CW, Bae HB, Kim SJ. Effect of ulinastatin on cytokine reaction during gastrectomy. Korean J Anesthesiol. 2010; 58:334–337. PMID: 20508788.
Article7. Cao LJ, Wang J, Hao PP, Sun CL, Chen YG. Effects of ulinastatin, a urinary trypsin inhibitor, on synaptic plasticity and spatial memory in a rat model of cerebral ischemia/reperfusion injury. Chin J Physiol. 2011; 54:435–442. PMID: 22229512.8. Shu Y, Yang Y, Qiu W, Lu Z, Li Y, Bao J, et al. Neuroprotection by ulinastatin in experimental autoimmune encephalomyelitis. Neurochem Res. 2011; 36:1969–1977. PMID: 21667278.
Article9. Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992; 50:355–363. PMID: 1333581.
Article10. Chung JM, Kim HK, Chung K. Segmental spinal nerve ligation model of neuropathic pain. Methods Mol Med. 2004; 99:35–45. PMID: 15131327.
Article11. Dogrul A, Ossipov MH, Lai J, Malan TP Jr, Porreca F. Peripheral and spinal antihyperalgesic activity of SIB-1757, a metabotropic glutamate receptor (mGLUR(5)) antagonist, in experimental neuropathic pain in rats. Neurosci Lett. 2000; 292:115–118. PMID: 10998562.
Article12. Wang X, Xue Q, Yan F, Li L, Liu J, Li S, et al. Ulinastatin as a neuroprotective and anti-inflammatory agent in infant piglets model undergoing surgery on hypothermic low-flow cardiopulmonary bypass. Paediatr Anaesth. 2013; 23:209–216. PMID: 23384299.
Article13. Zhu WJ, Yamanaka H, Obata K, Dai Y, Kobayashi K, Kozai T, et al. Expression of mRNA for four subtypes of the proteinase-activated receptor in rat dorsal root ganglia. Brain Res. 2005; 1041:205–211. PMID: 15829229.
Article14. Lee HL, Lee KM, Son SJ, Hwang SH, Cho HJ. Temporal expression of cytokines and their receptors mRNAs in a neuropathic pain model. Neuroreport. 2004; 15:2807–2811. PMID: 15597059.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Urinary trypsin inhibitor: miraculous medicine in many surgical situations?
- Effects of Trypsin, Collagenase and Dimethyl Sulfoxide on Dissociation of Rat Heart Cells
- Tumor-associated trypsin inhibitor(TATI) in gynecologic tumors
- Comparison of Antiallodynic Effects between Intrathecal Cholinesterase Inhibitors and NMDA Antagonists on Two Neuropathic Pain Rat Models
- The Combined Antiallodynic Effect of Gabapentin and Milnacipran in a Rat Neuropathic Pain Model