Korean J Physiol Pharmacol.
2015 Jan;19(1):35-42. 10.4196/kjpp.2015.19.1.35.
Isolation and In Vitro Culture of Vascular Endothelial Cells from Mice
- Affiliations
-
- 1Department of Physiology, School of Medicine, Ewha Womans University, Seoul 157-710, Korea. shsuh@ewha.ac.kr
- 2Department of Thoracic & Cardiovascular Surgery and Ewha Womans University Global Top 5 Research Program, School of Medicine, Ewha Womans University, Seoul 157-710, Korea.
- KMID: 2071817
- DOI: http://doi.org/10.4196/kjpp.2015.19.1.35
Abstract
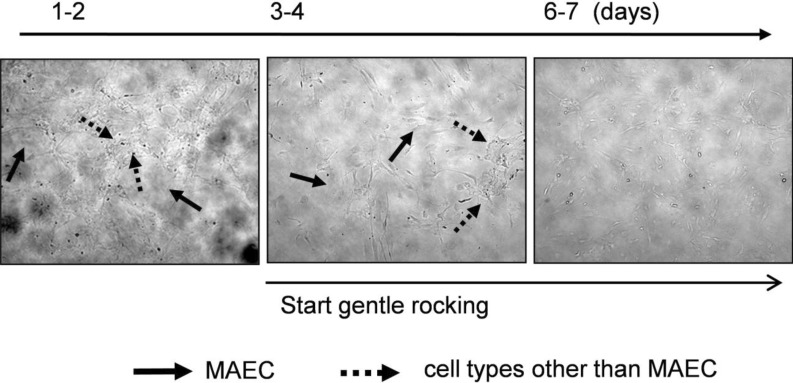
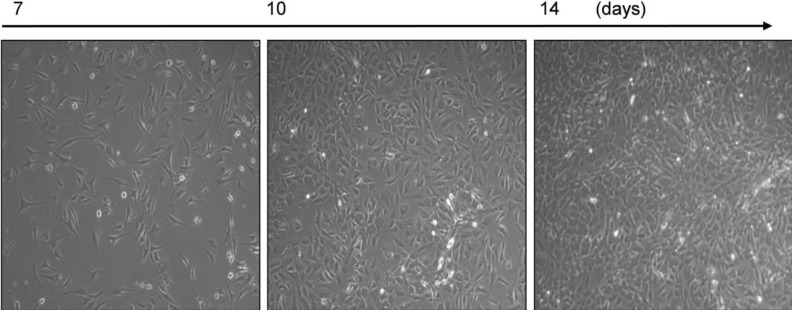
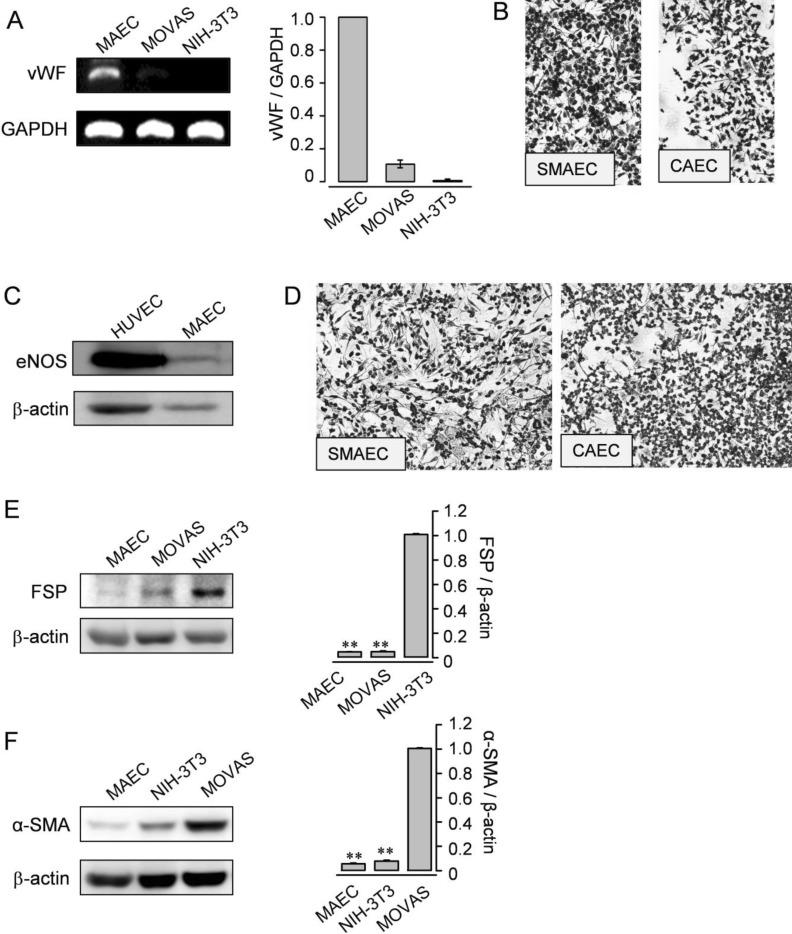
- In cardiovascular disorders, understanding of endothelial cell (EC) function is essential to elucidate the disease mechanism. Although the mouse model has many advantages for in vivo and in vitro research, efficient procedures for the isolation and propagation of primary mouse EC have been problematic. We describe a high yield process for isolation and in vitro culture of primary EC from mouse arteries (aorta, braches of superior mesenteric artery, and cerebral arteries from the circle of Willis). Mouse arteries were carefully dissected without damage under a light microscope, and small pieces of the vessels were transferred on/in a Matrigel matrix enriched with endothelial growth supplement. Primary cells that proliferated in Matrigel were propagated in advanced DMEM with fetal calf serum or platelet-derived serum, EC growth supplement, and heparin. To improve the purity of the cell culture, we applied shearing stress and anti-fibroblast antibody. EC were characterized by a monolayer cobble stone appearance, positive staining with acetylated low density lipoprotein labeled with 1,1'-dioctadecyl-3,3,3',3'-tetramethyl-indocarbocyanine perchlorate, RT-PCR using primers for von-Willebrand factor, and determination of the protein level endothelial nitric oxide synthase. Our simple, efficient method would facilitate in vitro functional investigations of EC from mouse vessels.
Keyword
MeSH Terms
Figure
Reference
-
1. Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003; 9:653–660. PMID: 12778163.
Article2. Choi S, Kim JA, Na HY, Kim JE, Park S, Han KH, Kim YJ, Suh SH. NADPH oxidase 2-derived superoxide downregulates endothelial KCa3.1 in preeclampsia. Free Radic Biol Med. 2013; 57:10–21. PMID: 23261940.
Article3. Choi S, Kim JA, Na HY, Cho SE, Park S, Jung SC, Suh SH. Globotriaosylceramide induces lysosomal degradation of endothelial KCa3.1 in fabry disease. Arterioscler Thromb Vasc Biol. 2014; 34:81–89. PMID: 24158513.4. Park S, Kim JA, Joo KY, Choi S, Choi EN, Shin JA, Han KH, Jung SC, Suh SH. Globotriaosylceramide leads to K(Ca)3.1 channel dysfunction: a new insight into endothelial dysfunction in Fabry disease. Cardiovasc Res. 2011; 89:290–299. PMID: 20971723.
Article5. Ni CW, Kumar S, Ankeny CJ, Jo H. Development of immortalized mouse aortic endothelial cell lines. Vasc Cell. 2014; 6:7. PMID: 24690145.
Article6. Nishiyama T, Mishima K, Ide F, Yamada K, Obara K, Sato A, Hitosugi N, Inoue H, Tsubota K, Saito I. Functional analysis of an established mouse vascular endothelial cell line. J Vasc Res. 2007; 44:138–148. PMID: 17215585.
Article7. Kobayashi M, Inoue K, Warabi E, Minami T, Kodama T. A simple method of isolating mouse aortic endothelial cells. J Atheroscler Thromb. 2005; 12:138–142. PMID: 16020913.
Article8. Magid R, Martinson D, Hwang J, Jo H, Galis ZS. Optimization of isolation and functional characterization of primary murine aortic endothelial cells. Endothelium. 2003; 10:103–109. PMID: 12791518.
Article9. Kevil CG, Bullard DC. In vitro culture and characterization of gene targeted mouse endothelium. Acta Physiol Scand. 2001; 173:151–157. PMID: 11678738.
Article10. Suh SH, Vennekens R, Manolopoulos VG, Freichel M, Schweig U, Prenen J, Flockerzi V, Droogmans G, Nilius B. Characterisation of explanted endothelial cells from mouse aorta: electrophysiology and Ca2+ signalling. Pflugers Arch. 1999; 438:612–620. PMID: 10555557.11. Dong QG, Bernasconi S, Lostaglio S, De Calmanovici RW, Martin-Padura I, Breviario F, Garlanda C, Ramponi S, Mantovani A, Vecchi A. A general strategy for isolation of endothelial cells from murine tissues. Characterization of two endothelial cell lines from the murine lung and subcutaneous sponge implants. Arterioscler Thromb Vasc Biol. 1997; 17:1599–1604. PMID: 9301641.12. Voyta JC, Via DP, Butterfield CE, Zetter BR. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984; 99:2034–2040. PMID: 6501412.
Article13. Gerritsen ME, Shen CP, McHugh MC, Atkinson WJ, Kiely JM, Milstone DS, Luscinskas FW, Gimbrone MA Jr. Activationdependent isolation and culture of murine pulmonary microvascular endothelium. Microcirculation. 1995; 2:151–163. PMID: 7497167.
Article14. Sahagun G, Moore SA, Fabry Z, Schelper RL, Hart MN. Purification of murine endothelial cell cultures by flow cytometry using fluorescein-labeled griffonia simplicifolia agglutinin. Am J Pathol. 1989; 134:1227–1232. PMID: 2757116.15. Alroy J, Goyal V, Skutelsky E. Lectin histochemistry of mammalian endothelium. Histochemistry. 1987; 86:603–607. PMID: 3610672.
Article16. Gerritsen ME, Shen CP, Atkinson WJ, Padgett RC, Gimbrone MA Jr, Milstone DS. Microvascular endothelial cells from E-selectin-deficient mice form tubes in vitro. Lab Invest. 1996; 75:175–184. PMID: 8765318.17. Barkalow FJ, Goodman MJ, Mayadas TN. Cultured murine cerebral microvascular endothelial cells contain von Willebrand factor-positive Weibel-Palade bodies and support rapid cytokine-induced neutrophil adhesion. Microcirculation. 1996; 3:19–28. PMID: 8846268.18. Barkalow FJ, Goodman MJ, Gerritsen ME, Mayadas TN. Brain endothelium lack one of two pathways of P-selectin-mediated neutrophil adhesion. Blood. 1996; 88:4585–4593. PMID: 8977250.
Article19. Su X, Sorenson CM, Sheibani N. Isolation and characterization of murine retinal endothelial cells. Mol Vis. 2003; 9:171–178. PMID: 12740568.20. Reiss Y, Hoch G, Deutsch U, Engelhardt B. T cell interaction with ICAM-1-deficient endothelium in vitro: essential role for ICAM-1 and ICAM-2 in transendothelial migration of T cells. Eur J Immunol. 1998; 28:3086–3099. PMID: 9808177.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In Vitro Culture of Endothelial Cell and Smooth Muscle Cell for Studying Vascular Diseases
- Effects of Dimethyl Sulfoxide on the Differentiation of Myocardial and Endothelial Cells
- Comparative Evaluation for Potential Differentiation of Endothelial Progenitor Cells and Mesenchymal Stem Cells into Endothelial-Like Cells
- An Effective Isolation of the Vascular Endothelial and Smooth Muscle Cells from the Mouse Aorta
- Culture of the Human Glomerular Endothelial Cells