Korean J Physiol Pharmacol.
2010 Oct;14(5):305-310. 10.4196/kjpp.2010.14.5.305.
Response of I(Kr) and hERG Currents to the Antipsychotics Tiapride and Sulpiride
- Affiliations
-
- 1Department of Physiology, Institute of Bioscience and Biotechnology, Kangwon National University School of Medicine, Chuncheon 200-701, Korea. suhyunjo@kangwon.ac.kr
- 2Department of Life Science, Pohang University of Science and Technology, Pohang 790-784, Korea.
- KMID: 2071698
- DOI: http://doi.org/10.4196/kjpp.2010.14.5.305
Abstract
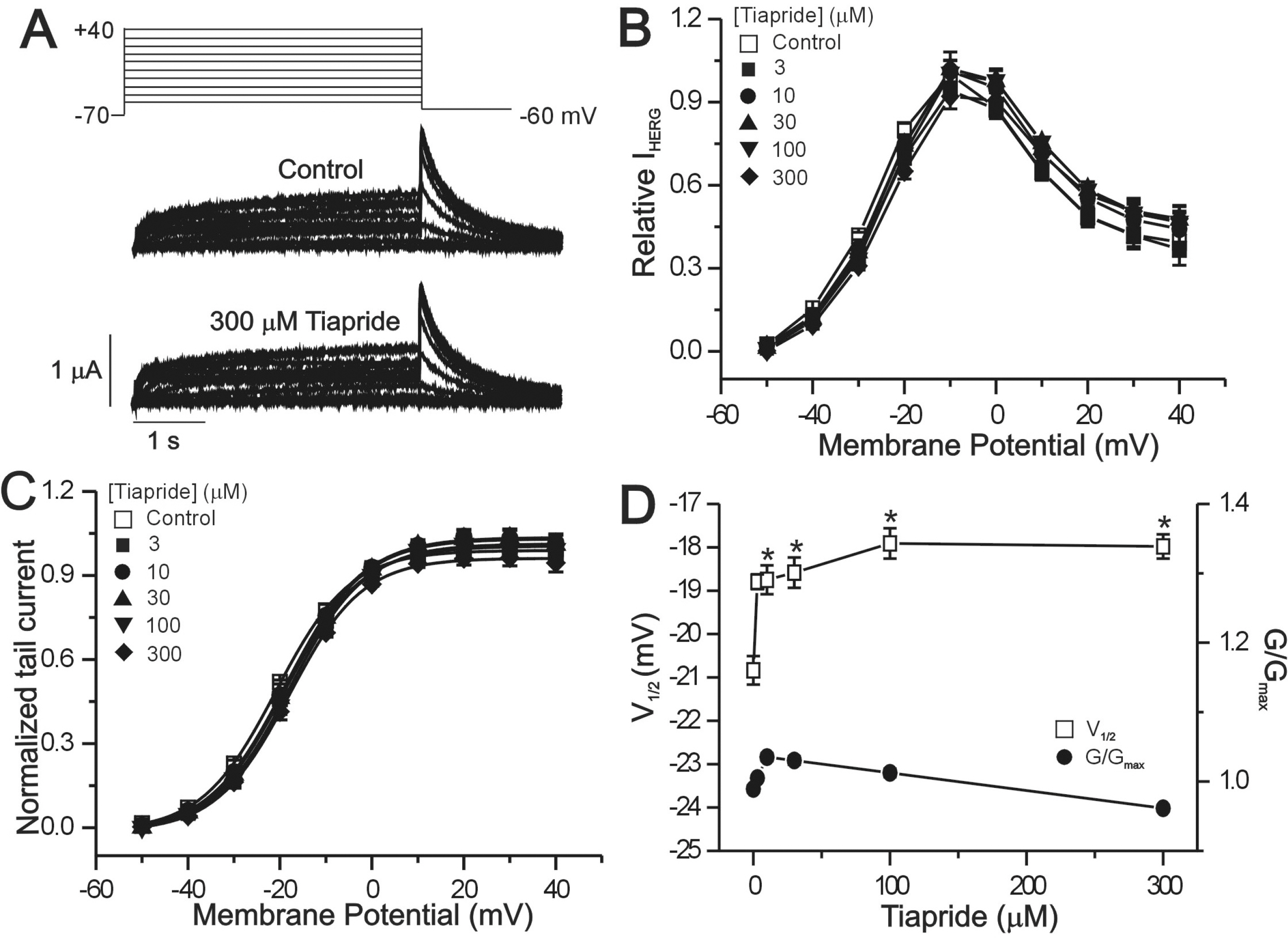
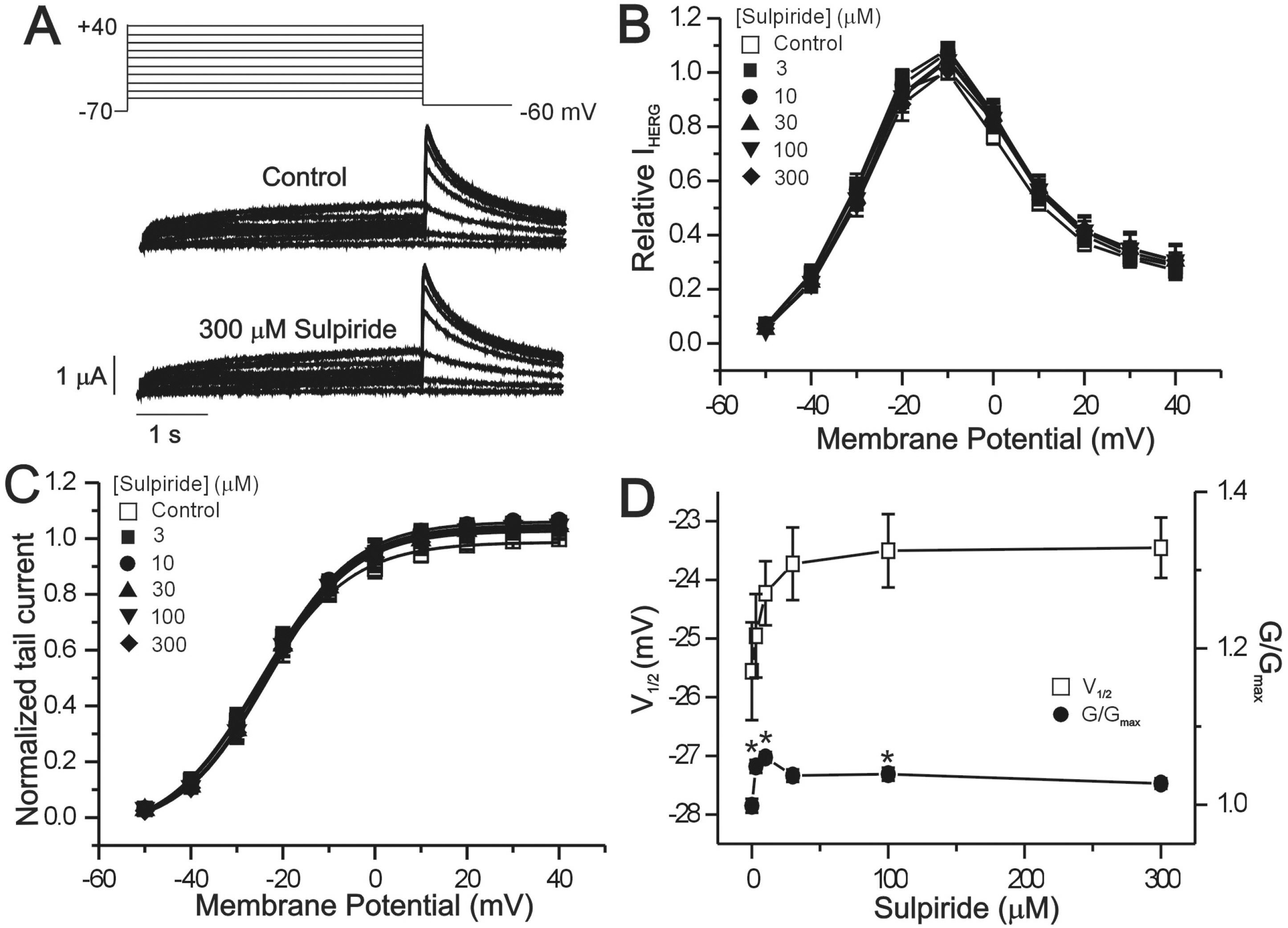
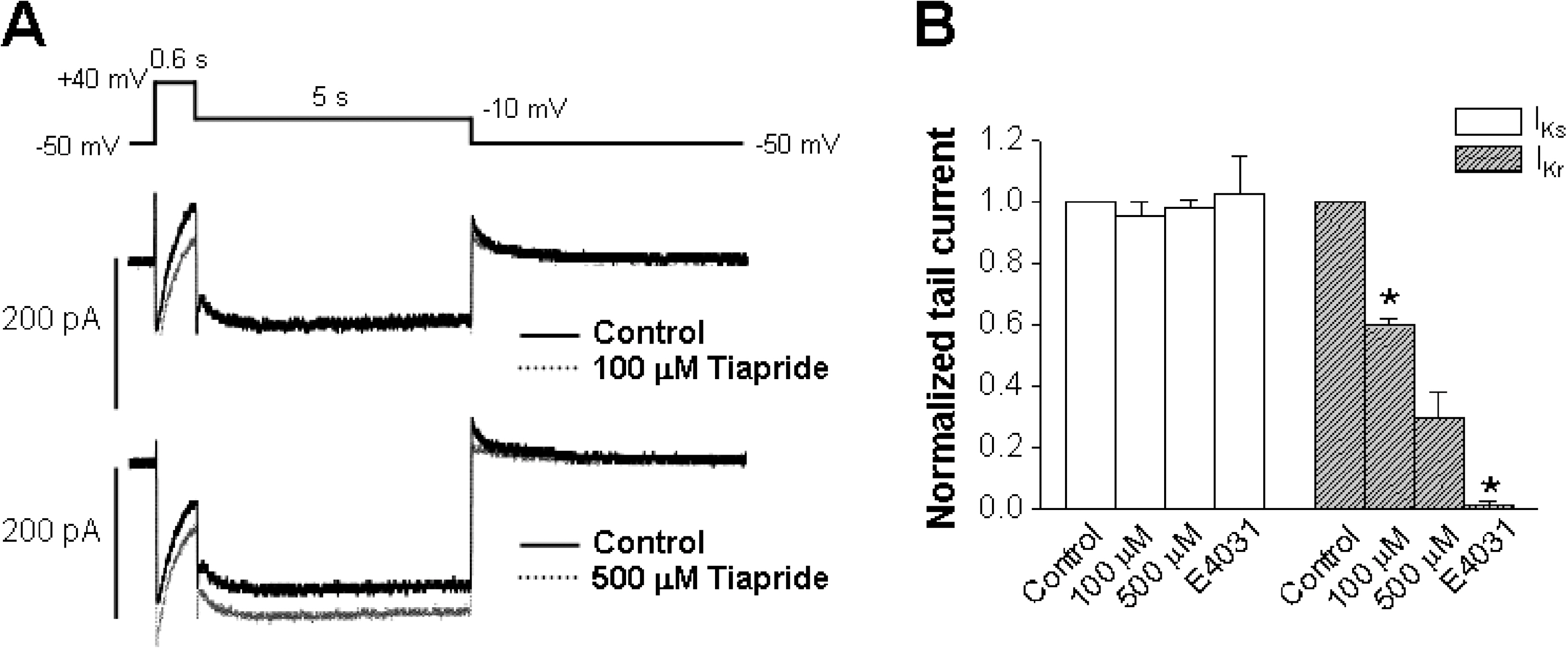
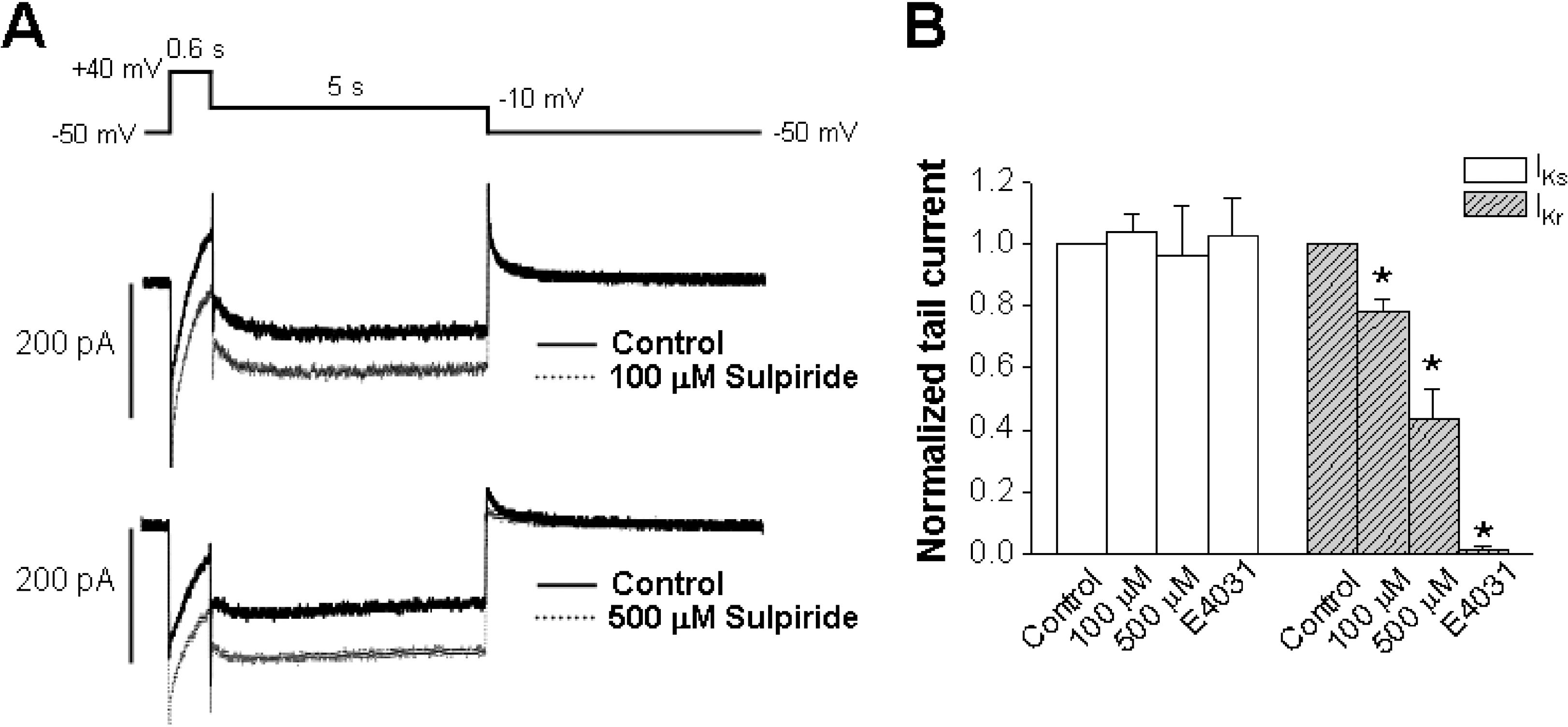
- The human ether-a-go-go-related gene (hERG) channel is important for repolarization in human myocardium and is a common target for drugs that prolong the QT interval. We studied the effects of two antipsychotics, tiapride and sulpiride, on hERG channels expressed in Xenopus oocytes and also on delayed rectifier K+ currents in guinea pig cardiomyocytes. Neither the amplitude of the hERG outward currents measured at the end of the voltage pulse, nor the amplitude of hERG tail currents, showed any concentration-dependent changes with either tiapride or sulpiride (3~300 micrometer). However, our findings did show that tiapride increased the potential for half-maximal activation (V(1/2)) of HERG at 10~300 micrometer, whereas sulpiride increased the maximum conductance (G(max)) at 3, 10 and 100 micrometer. In guinea pig ventricular myocytes, bath applications of 100 and 500 micrometer tiapride at 36degrees C blocked rapidly activating delayed rectifier K+ current (I(Kr)) by 40.3% and 70.0%, respectively. Also, sulpiride at 100 and 500 micrometer blocked I(Kr) by 38.9% and 76.5%, respectively. However, neither tiapride nor sulpiride significantly affected the slowly activating delayed rectifier K+ current (I(Ks)) at the same concentrations. Our findings suggest that the concentrations of the antipsychotics required to evoke a 50% inhibition of IKr are well above the reported therapeutic plasma concentrations of free and total compound.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Stöllberger C, Huber JO, Finsterer J. Antipsychotic drugs and QT prolongation. Int Clin Psychopharmacol. 2005; 20:243–251.
Article2. Hennessy S, Bilker WB, Knauss JS, Margolis DJ, Kimmel SE, Reynolds RF, Glasser DB, Morrison MF, Strom BL. Cardiac arrest and ventricular arrhythmia in patients taking antipsychotic drugs: cohort study using administrative data. BMJ. 2002; 325:1070–1074.
Article3. Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, Murray KT. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001; 58:1161–1167.
Article4. Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SHL. Thioridazine and sudden unexplained death in psychiatric in-patients. Br J Psychiatry. 2002; 180:515–522.
Article5. Straus SM, Bleumink GS, Dieleman JP, van der Lei J, 't Jong GW, Kingma JH, Sturkenboom MC, Stricker BH. Antipsychotics and the risk of sudden cardiac death. Arch Intern Med. 2004; 164:1293–1297.
Article6. Steele JW, Faulds D, Sorkin EM. Tiapride. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in geriatric agitation. Drugs Aging. 1993; 3:460–478.7. Mucci A, Nolfe G, Maj M. Levosulpiride: a review of its clinical use in psychiatry. Pharmacol Res. 1995; 31:95–101.
Article8. Capel MM, Colbridge MG, Henry JA. Overdose profiles of new antipsychotic agents. Int J Neuropsychopharmacol. 2000; 3:51–54.
Article9. Huang BH, Hsia CP, Chen CY. Sulpiride induced torsade de pointes. Int J Cardiol. 2007; 118:e100–e102.
Article10. Iglesias E, Esteban E, Zabala S, Gascon A. Tiapride-induced torsade de pointes. Am J Med. 2000; 109:509.
Article11. Lin CH, Chen MC, Wang SY, Lin CY. Predictive factors for QTc prolongation in schizophrenic patients taking antipsychotics. J Formos Med Assoc. 2004; 103:437–441.12. Su KP, Shen WW, Chuang CL, Chen KP, Chen CC. A pilot cross-over design study on QTc interval prolongation associated with sulpiride and haloperidol. Schizophr Res. 2003; 59:93–94.
Article13. Tie H, Walker BD, Valenzuela SM, Breit SN, Campbell TJ. The Heart of Psychotropic drug therapy. Lancet. 2000; 355:1825.
Article14. Choi SY, Koh YS, Jo SH. Inhibition of human ether-a-go-go-related gene K+ channel and IKr of guinea pig cardiomyocytes by antipsychotic drug trifluoperazine. J Pharmacol Exp Ther. 2005; 313:888–895.15. Zhou Z, Gong Q, Ye B, Fan Z, Makielski JC, Robertson GA, January CT. Properties of HERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys J. 1998; 74:230–241.
Article16. Heath BM, Terrar DA. Separation of the components of the delayed rectifier potassium current using selective blockers of IKr and IKs in guinea-pig isolated ventricular myocytes. Exp Physiol. 1996; 81:587–603.
Article17. Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990; 96:195–215.18. Malik M. Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol. 2001; 12:411–420.
Article19. Malik M, Camm AJ. Evaluation of drug-induced QT interval prolongation: implications for drug approval and labeling. Drug Saf. 2001; 24:323–351.20. Sugiyama A, Satoh Y, Shiina H, Takeda S, Hashimoto K. Torsadegenic action of the antipsychotic drug sulpiride assessed using in vivo canine models. J Cardiovasc Pharmacol. 2002; 40:235–245.
Article21. Lenhard G, Kieferndorf U, Berner G, Vogtle-Junkert U, Wagener HH. The importance of pharmacokinetic data on sulpiride: results of a bioequivalence study of two sulpiride 200 mg preparations following oral administration. Int J Clin Pharmacol Ther Toxicol. 1991; 29:231–237.22. Bressolle F, Bres J, Blanchin MD, Gomeni R. Sulpiride pharmacokinetics in humans after intramuscular administration at three dose levels. J Pharm Sci. 1984; 73:1128–1136.23. Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, Siegl PK, Strang I, Sullivan AT, Wallis R, Camm AJ, Hammond TG. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003; 58:32–45.
Article24. Kiehn J, Lacerda AE, Wible B, Brown AM. Molecular physiology and pharmacology of HERG. Single-channel currents and block by dofetilide. Circulation. 1996; 94:2572–2579.25. Thomas D, Wendt-Nordahl G, Rockl K, Ficker E, Brown AM, Kiehn J. High-affinity blockade of human ether-a-go-go-related gene human cardiac potassium channels by the novel antiarrhythmic drug BRL-32872. J Pharmacol Exp Ther. 2001; 297:753–761.26. Madeja M, Musshoff U, Speckmann EJ. Follicular tissues reduce drug effects on ion channels in oocytes of Xenopus laevis. Eur J Neurosci. 1997; 9:599–604.
Article27. Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, Keating MT, Goldstein SA. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999; 97:175–187.
Article28. Curtis LH, Østbye T, Sendersky V, Hutchison S, Allen LaPointe NM, Al-Khatib SM, Usdin Yasuda S, Dans PE, Wright A, Califf RM, Woosley RL, Schulman KA. Prescription of QT-prolonging drugs in a cohort of about 5 million outpatients. Am J Med. 2003; 114:135–141.
Article29. Silvestre JS, Prous JR. Comparative evaluation of hERG potassium channel blockade by antipsychotics. Methods Find Exp Clin Pharmacol. 2007; 29:457–465.30. Lee HA, Kim KS, Park SJ, Kim EJ. Cellular mechanism of the QT prolongation induced by sulpiride. Int J Toxicol. 2009; 28:207–212.
Article31. Ito K, Nakazawa K, Koizumi S, Liu M, Takeuchi K, Hashimoto T, Ohno Y, Inoue K. Inhibition by antipsychotic drugs of L-type Ca2+ channel current in PC12 cells. Eur J Pharmacol. 1996; 314:143–150.32. Nakazawa K, Ito K, Koizumi S, Ohno Y, Inoue K. Characterization of inhibition by haloperidol and chlorpromazine of a voltage-activated K+ current in rat phaeochromocytoma cells. Br J Pharmacol. 1995; 116:2603–2610.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acepromazine inhibits hERG potassium ion channels expressed in human embryonic kidney 293 cells
- The clinical effects and serum concentrations of sulpiride ib positive and negative symptom schizophrenics
- Recent Trends of Antipsychotics Polypharmacy in Schizophrenia
- Block of hERG K+ Channel by Classic Histamine H1 Receptor Antagonist Chlorpheniramine
- Clinical Experience of Clozapine Discontinuation: Comparison between Sulpiride and Thioridazine Switch Group