J Korean Neurosurg Soc.
2015 Jan;57(1):6-11. 10.3340/jkns.2015.57.1.6.
The Neuromodulation of Neuropathic Pain by Measuring Pain Response Rate and Pain Response Duration in Animal
- Affiliations
-
- 1Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea. jchang@yuhs.ac
- 2Department of Neurosurgery, Yonsei University College of Medicine, Seoul, Korea.
- 3School of Electrical Engineering and Computer Science, Seoul National University, Seoul, Korea.
- 4Nano Bioelectronics and System Research Center, Seoul National University, Seoul, Korea.
- 5Inter-University Semiconductor Research Center, Seoul National University, Seoul, Korea.
- KMID: 2067084
- DOI: http://doi.org/10.3340/jkns.2015.57.1.6
Abstract
OBJECTIVE
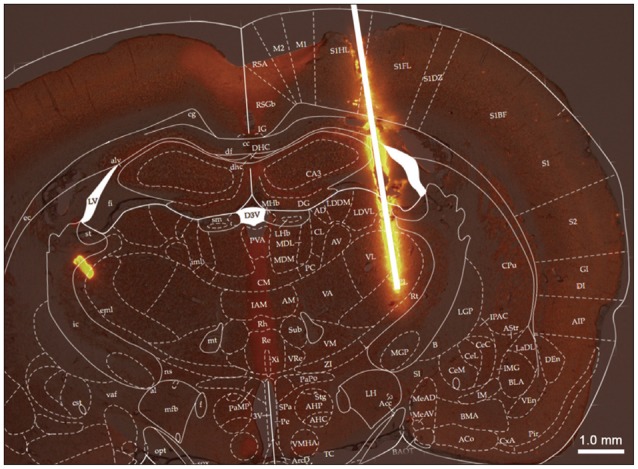
Neuropathic pain causes patients feel indescribable pain. Deep Brain Stimulation (DBS) is one of the treatment methods in neuropathic pain but the action mechanism is still unclear. To study the effect and mechanism of analgesic effects from DBS in neuropathic pain and to enhance the analgesic effect of DBS, we stimulated the ventral posterolateral nucleus (VPL) in rats.
METHODS
To observe the effect from VPL stimulation, we established 3 groups : normal group (Normal group), neuropathic pain group (Pain group) and neuropathic pain+DBS group (DBS group). Rats in DBS group subjected to electrical stimulation and the target is VPL.
RESULTS
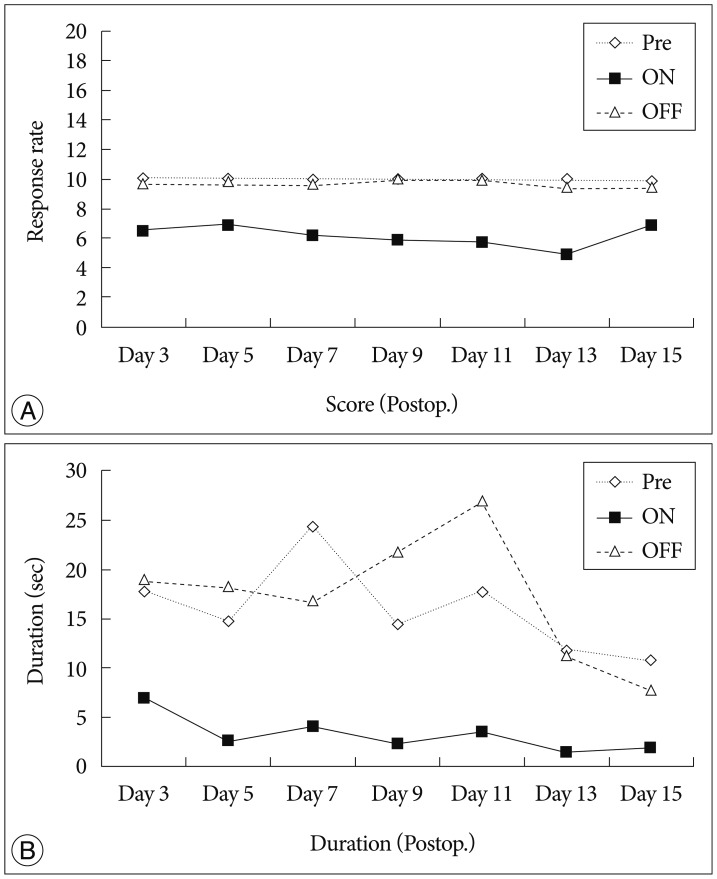
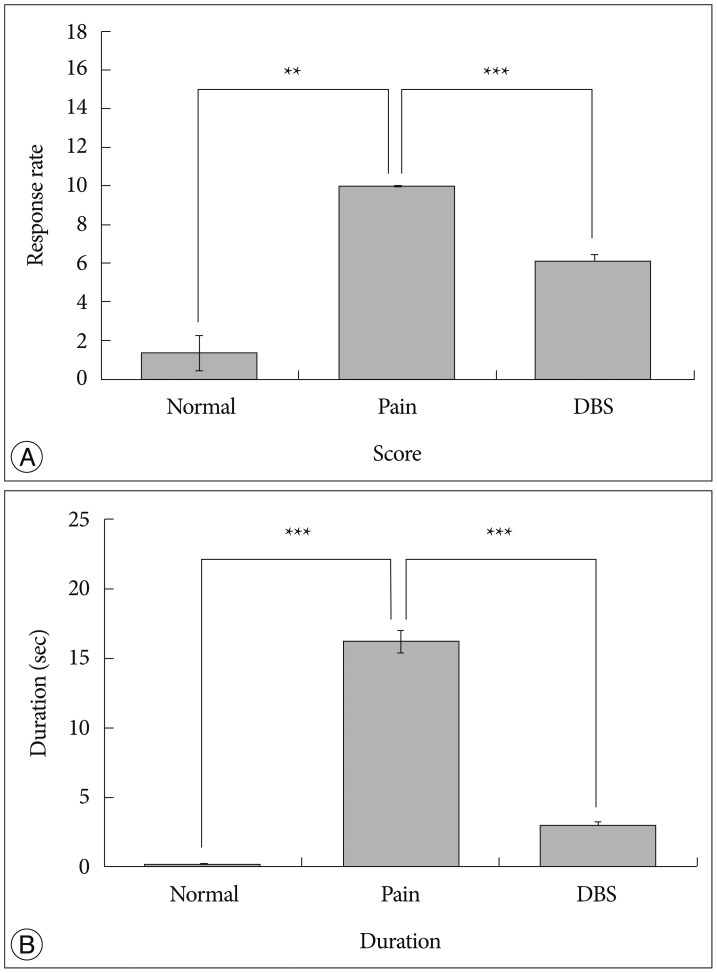
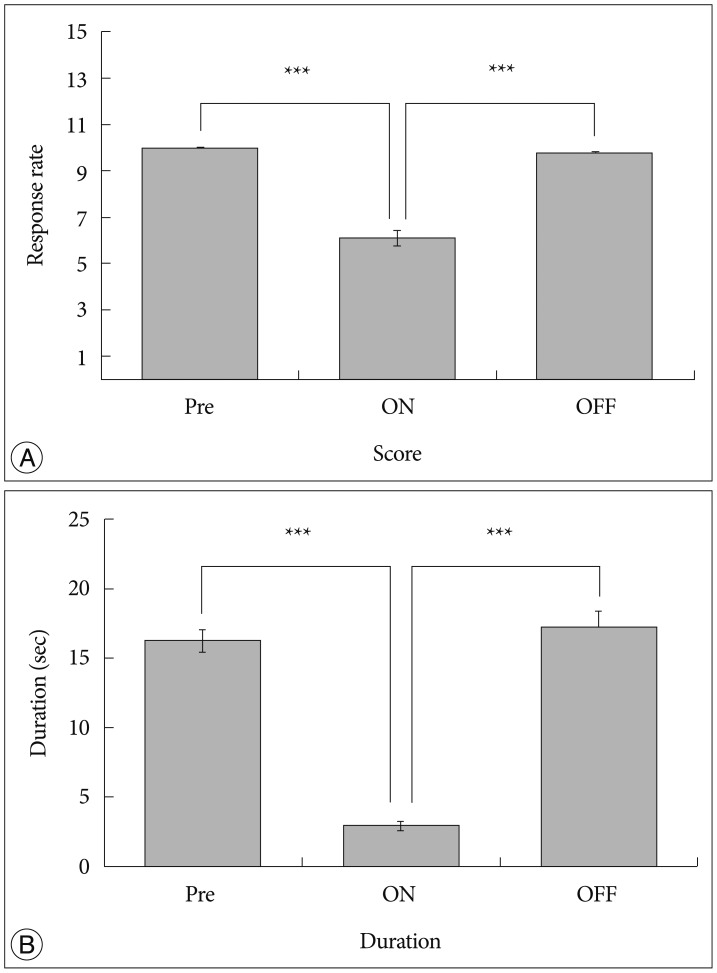
We observed the behavioral changes by DBS in VPL (VPL-DBS) on neuropathic pain rats. In our study, the pain score which is by conventional test method was effectively decreased. In specific, the time of showing withdrawal response from painful stimulation which is not used measuring method in our animal model was also decreased by DBS.
CONCLUSION
The VPL is an effective target on pain modulation. Specifically we could demonstrate changes of pain response duration which is not used, and it was also significantly meaningful. We thought that this study would be helpful in understanding the relation between VPL-DBS and neuropathic pain.
MeSH Terms
Figure
Reference
-
1. Blomstedt P, Sandvik U, Fytagoridis A, Tisch S. The posterior subthalamic area in the treatment of movement disorders : past, present, and future. Neurosurgery. 2009; 64:1029–1038. discussion 1038-1042. PMID: 19487881.2. Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994; 59:369–376. PMID: 7708411.
Article3. DaSilva AF, Granziera C, Snyder J, Hadjikhani N. Thickening in the somatosensory cortex of patients with migraine. Neurology. 2007; 69:1990–1995. PMID: 18025393.
Article4. Deniau JM, Degos B, Bosch C, Maurice N. Deep brain stimulation mechanisms : beyond the concept of local functional inhibition. Eur J Neurosci. 2010; 32:1080–1091. PMID: 21039947.
Article5. Dowdall T, Robinson I, Meert TF. Comparison of five different rat models of peripheral nerve injury. Pharmacol Biochem Behav. 2005; 80:93–108. PMID: 15652385.
Article6. Gubellini P, Salin P, Kerkerian-Le Goff L, Baunez C. Deep brain stimulation in neurological diseases and experimental models : from molecule to complex behavior. Prog Neurobiol. 2009; 89:79–123. PMID: 19559747.
Article7. Hubscher CH, Johnson RD. Chronic spinal cord injury induced changes in the responses of thalamic neurons. Exp Neurol. 2006; 197:177–188. PMID: 16266704.
Article8. Kahane P, Depaulis A. Deep brain stimulation in epilepsy : what is next. Curr Opin Neurol. 2010; 23:177–182. PMID: 20125010.9. Kim J, Kim J, Min KS, Lee SE, Kim SJ, Chang JW. VPL-DBS on neuropathic pain rat model is effective in mechanical allodynia than cold allodynia. Neurol Sci. 2012; 33:1265–1270. PMID: 22562402.
Article10. Kringelbach ML, Jenkinson N, Green AL, Owen SL, Hansen PC, Cornelissen PL. Deep brain stimulation for chronic pain investigated with magnetoencephalography. Neuroreport. 2007; 18:223–228. PMID: 17314661.
Article11. Lee BH, Won R, Baik EJ, Lee SH, Moon CH. An animal model of neuropathic pain employing injury to the sciatic nerve branches. Neuroreport. 2000; 11:657–661. PMID: 10757496.
Article12. Lucas JM, Ji Y, Masri R. Motor cortex stimulation reduces hyperalgesia in an animal model of central pain. Pain. 2011; 152:1398–1407. PMID: 21396776.
Article13. Merskey H, Bogduk N. Classification of chronic pain : Descriptions of chronic pain syndromes and definitions of pain terms. ed 2. Seattle: IASP Press;1994.14. Nandi D, Aziz TZ. Deep brain stimulation in the management of neuropathic pain and multiple sclerosis tremor. J Clin Neurophysiol. 2004; 21:31–39. PMID: 15097292.
Article15. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. ed 4. New York: Academic Press;1988.16. Perlmutter JS, Mink JW. Deep brain stimulation. Annu Rev Neurosci. 2006; 29:229–257. PMID: 16776585.
Article17. Pickering AE, Thornton SR, Love-Jones SJ, Steeds C, Patel NK. Analgesia in conjunction with normalisation of thermal sensation following deep brain stimulation for central post-stroke pain. Pain. 2009; 147:299–304. PMID: 19833434.
Article18. Seminowicz DA, Laferriere AL, Millecamps M, Yu JS, Coderre TJ. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. Neuroimage. 2009; 47:1007–1014. PMID: 19497372.
Article19. Shirvalkar P, Seth M, Schiff ND, Herrera DG. Cognitive enhancement with central thalamic electrical stimulation. Proc Natl Acad Sci U S A. 2006; 103:17007–17012. PMID: 17065322.
Article