Hanyang Med Rev.
2011 May;31(2):107-115. 10.7599/hmr.2011.31.2.107.
Animal Models for Orofacial Neuropathic Pain
- Affiliations
-
- 1Department of Oral Physiology, Kyungpook National University School of Dentistry, Daegu, Korea. dkahn@knu.ac.kr
- KMID: 2168187
- DOI: http://doi.org/10.7599/hmr.2011.31.2.107
Abstract
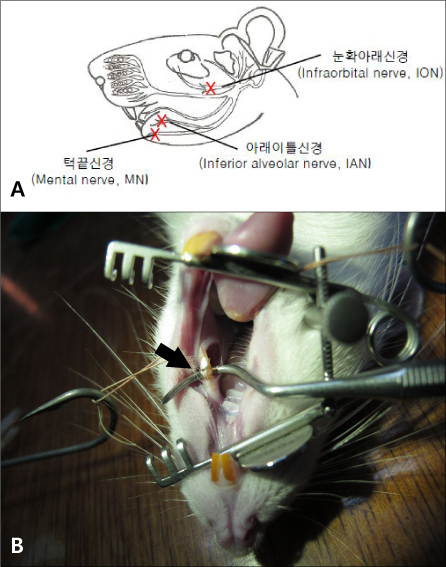
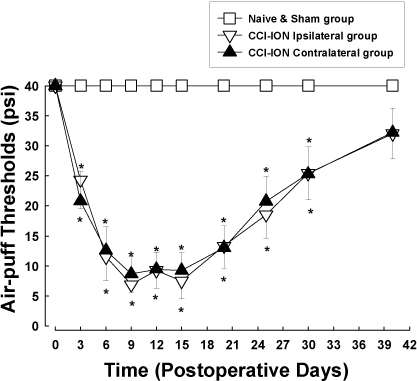
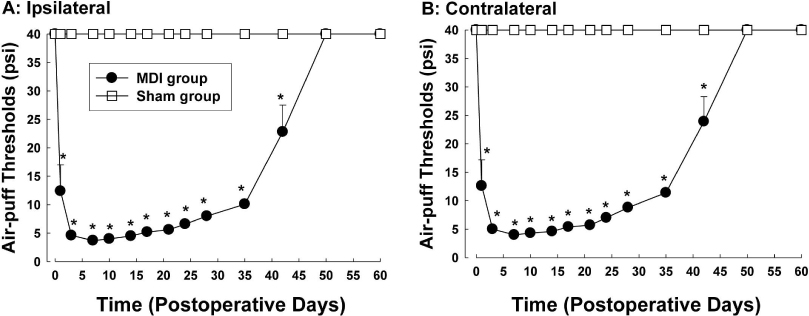
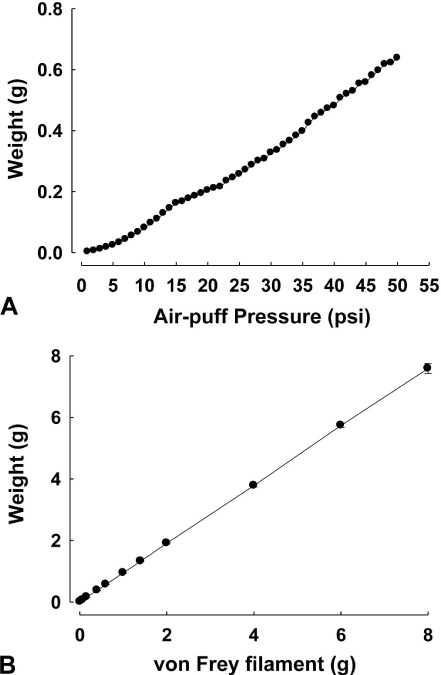
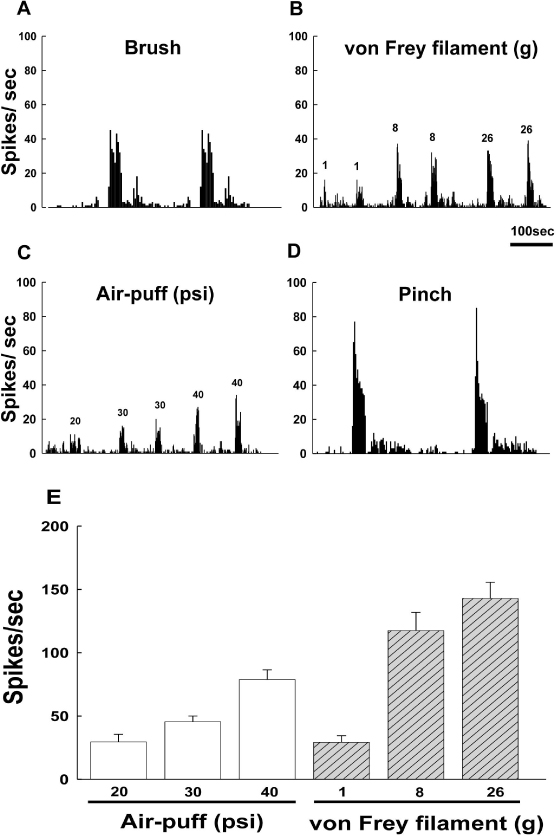
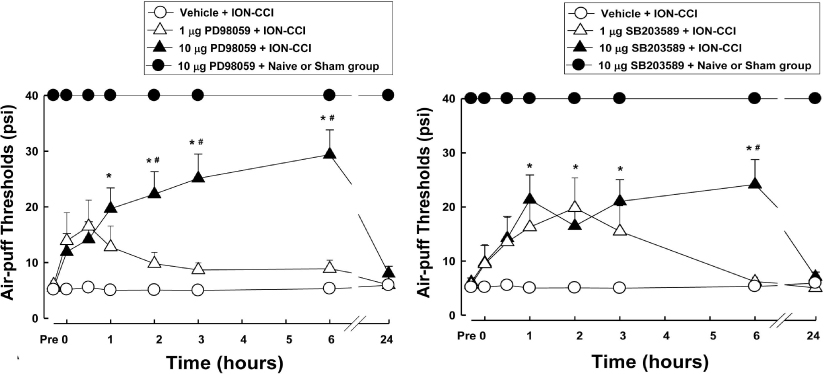
- Orofacial neuropathic pain is initiated by extraction of teeth or nerve injury from trauma in the trigeminal nerve that innervates the facial area. In the experiment, orofacial neuropathic pain usually occurred following injury of peripheral trigeminal nerve including infra-orbital nerve, inferior alveolar nerve, or mental nerve. In addition, pathology from trigeminal nerve root or ganglion is involved in orofacial neuropathic pain. This study introduced various animal models that help us study the underlying mechanisms of development or maintenance of orofacial neuropathic pain. One of the most typical symptoms of orofacial neuropathic pain is hypersensitivity to the innocuous mechanical stimuli. Our study presents a novel method to evaluate mechanical allodynia in rats with orofacial neuropathic pain. Recently, accumulate evidence support participation of central glial cells in the development or maintenance of orofacial neuropathic pain. Signaling molecules in glial cells also play an important role in neuropathic pain in the orofacial area.
Keyword
MeSH Terms
Figure
Reference
-
1. Scholz J, Woolf CJ. Can we conquer pain? Nat Neurosci. 2002. 5:5 Suppl. 1062–1067.
Article2. Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988. 33:87–107.
Article3. Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990. 43:205–218.
Article4. Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992. 50:355–363.
Article5. Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000. 87:149–158.
Article6. Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rats infraorbital nerve. J Neurosci. 1994. 14:2708–2723.
Article7. Lim EJ, Jeon HJ, Yang GY, Lee MK, Ju JS, Han SR, Ahn DK. Intracisternal administration of mitogen-activated protein kinase inhibitors reduced mechanical allodynia following chronic constriction injury of infraorbital nerve in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2007. 31:1322–1329.
Article8. Han SR, Yeo SP, Lee MK, Bae YC, Ahn DK. Early dexamethasone relieves trigeminal neuropathic pain. J Dent Res. 2010. 89:915–920.
Article9. Jannetta PJ. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg. 1967. 26:159–162.
Article10. Love S, Coakham HB. Trigeminal neuralgia: pathology and pathogenesis. Brain. 2001. 124:2347–2360.
Article11. Ahn DK, Lim EJ, Kim BC, Yang GY, Lee MK, Ju JS, Han SR, Bae YC. Compression of the trigeminal ganglion produces prolonged nociceptive behavior in rats. Eur J Pain. 2009. 13:568–575.
Article12. Ahn DK, Jung CY, Lee HJ, Choi HS, Ju JS, Bae YC. Peripheral glutamate receptors participate in interleukin-1beta-induced mechanical allodynia in the orofacial area of rats. Neurosci Lett. 2004. 357:203–206.
Article13. Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988. 32:77–88.
Article14. Ahn DK, Lee SY, Han SR, Ju JS, Yang GY, Lee MK, Youn DH, Bae YC. Intratrigeminal ganglionic injection of LPA causes neuropathic pain-like behavior and demyelination in rats. Pain. 2009. 146:114–120.
Article15. Park CK, Kim K, Jung SJ, Kim MJ, Ahn DK, Hong SD, Kim JS, Oh SB. Molecular mechanism for local anesthetic action of eugenol in the rat trigeminal system. Pain. 2009. 144:84–94.
Article16. Gosselin RD, Suter MR, Ji RR, Decosterd I. Glial cells and chronic pain. Neuroscientist. 2010. 16:519–531.
Article17. Beggs S, Salter MW. Stereological and somatotopic analysis of the spinal microglial response to peripheral nerve injury. Brain Behav Immun. 2007. 21:624–633.
Article18. Chang YW, Tan A, Saab C, Waxman S. Unilateral focal burn injury is followed by long-lasting bilateral allodynia and neuronal hyperexcitability in spinal cord dorsal horn. J Pain. 2010. 11:119–130.
Article19. Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in "small" glia. Trends Neurosci. 2005. 28:101–107.
Article20. Ji RR, Gereau RW 4th, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009. 60:135–148.
Article21. Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a threekinase module from yeast to human. Physiol Rev. 1999. 79:143–180.
Article22. Impey S, Obrietan K, Storm DR. Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron. 1999. 23:11–14.23. White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci U S A. 2007. 104:20151–20158.
Article24. Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001. 8:1–10.
Article25. Gao YJ, Ji RR. Activation of JNK pathway in persistent pain. Neurosci Lett. 2008. 437:180–183.
Article26. Terayama R, Fujisawa N, Yamaguchi D, Omura S, Ichikawa H, Sugimoto T. Differential activation of mitogen-activated protein kinases and glial cells in the trigeminal sensory nuclear complex following lingual nerve injury. Neurosci Res. 2011. 69:100–110.
Article27. Mei XP, Zhang H, Wang W, Wei YY, Zhai MZ, Xu LX, Li YQ. Inhibition of spinal astrocytic c-Jun N-terminal kinase (JNK) activation correlates with the analgesic effects of ketamine in neuropathic pain. J Neuroinflammation. 2011. 8:6.
Article28. Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci. 2004. 24:10211–10222.
Article29. Kommers T, Vinade L, Pereira C, Goncalves CA, Wofchuk S, Rodnight R. Regulation of the phosphorylation of glial fibrillary acidic protein (GFAP) by glutamate and calcium ions in slices of immature rat spinal cord: comparison with immature hippocampus. Neurosci Lett. 1998. 248:141–143.
Article30. Inoue A, Ikoma K, Morioka N, Kumagai K, Hashimoto T, Hide I, Nakata Y. Interleukin-1beta induces sub--stance P release from primary afferent neurons through the cyclooxygenase-2 system. J Neurochem. 1999. 73:2206–2213.31. Eroglu C. The role of astrocyte-secreted matricellular proteins in central nervous system development and function. J Cell Commun Signal. 2009. 3:167–176.
Article32. Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci. 2007. 8:206–220.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of Current Withdrawal Threshold on Evaluation of the Neuropathic Pain in Animal Model
- Animal Pain Models and Behavior Tests
- Clinical Scale for Neuropathic Pain
- The Neuromodulation of Neuropathic Pain by Measuring Pain Response Rate and Pain Response Duration in Animal
- Neuropathic Pain Model of Peripheral Neuropathies Mediated by Mutations of Glycyl-tRNA Synthetase