J Korean Neurosurg Soc.
2015 Jan;57(1):1-5. 10.3340/jkns.2015.57.1.1.
Development of an Ex Vivo Model for the Study of Cerebrovascular Function Utilizing Isolated Mouse Olfactory Artery
- Affiliations
-
- 1Department of Neurological Surgery, The Catholic University of Korea, Daejeon St. Mary's Hospital, Daejeon, Korea. hyungjin@catholic.ac.kr
- 2Hope Center for Neurological Disorders, Washington University School of Medicine, St. Louis, MO, USA.
- 3Department of Neurological Surgery, Washington University School of Medicine, St. Louis, MO, USA.
- 4Alzheimers Disease Research Center, Washington University School of Medicine, St. Louis, MO, USA.
- KMID: 2067083
- DOI: http://doi.org/10.3340/jkns.2015.57.1.1
Abstract
OBJECTIVE
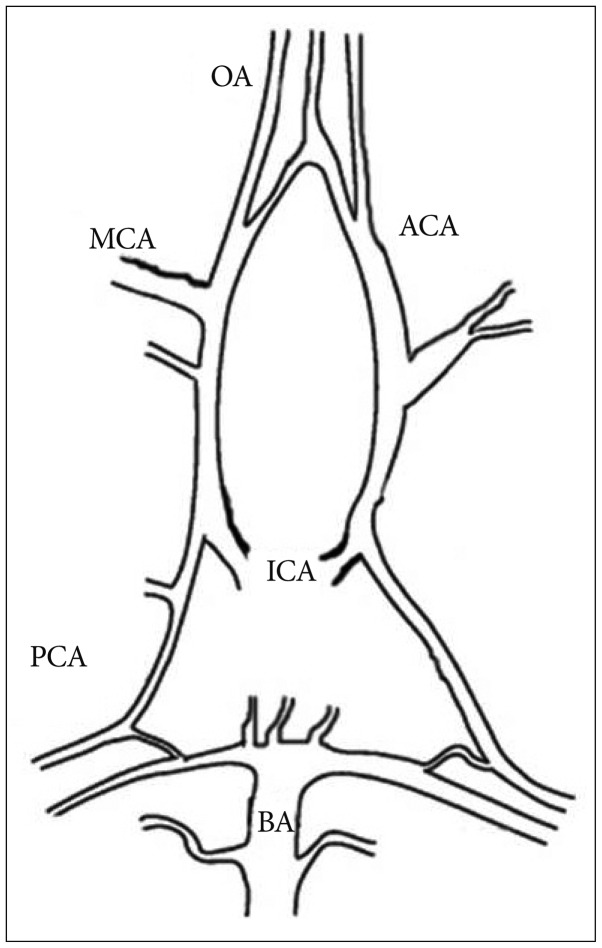
Cerebral vessels, such as intracerebral perforating arterioles isolated from rat brain, have been widely used as an ex vivo model to study the cerebrovascular function associated with cerebrovascular disorders and the therapeutic effects of various pharmacological agents. These perforating arterioles, however, have demonstrated differences in the vascular architecture and reactivity compared with a larger leptomeningeal artery which has been commonly implicated in cerebrovascular disease. In this study, therefore, we developed the method for studying cerebrovascular function utilizing the olfactory artery isolated from the mouse brain.
METHODS
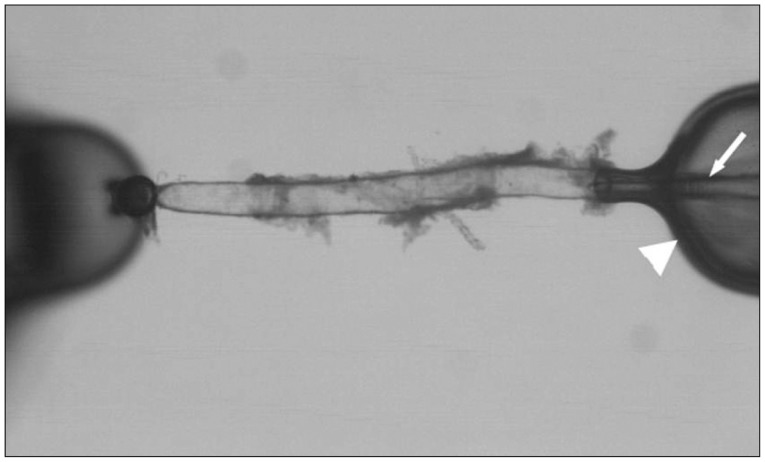
The olfactory artery (OA) was isolated from the C57/BL6 wild-type mouse brain. After removing connective tissues, one side of the isolated vessel segment (approximately -500 microm in length) was cannulated and the opposite end of the vessel was completely sealed while being viewed with an inverted microscope. After verifying the absence of pressure leakage, we examined the vascular reactivity to various vasoactive agents under the fixed intravascular pressure (60 mm Hg).
RESULTS
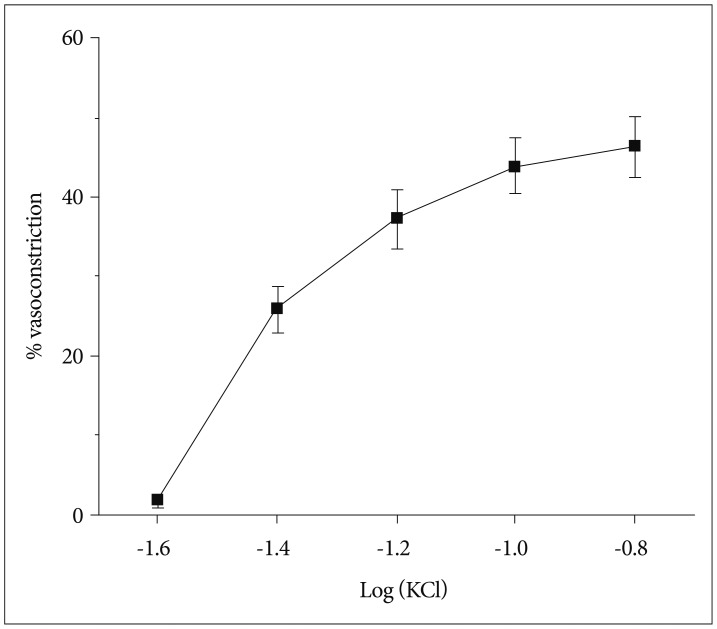
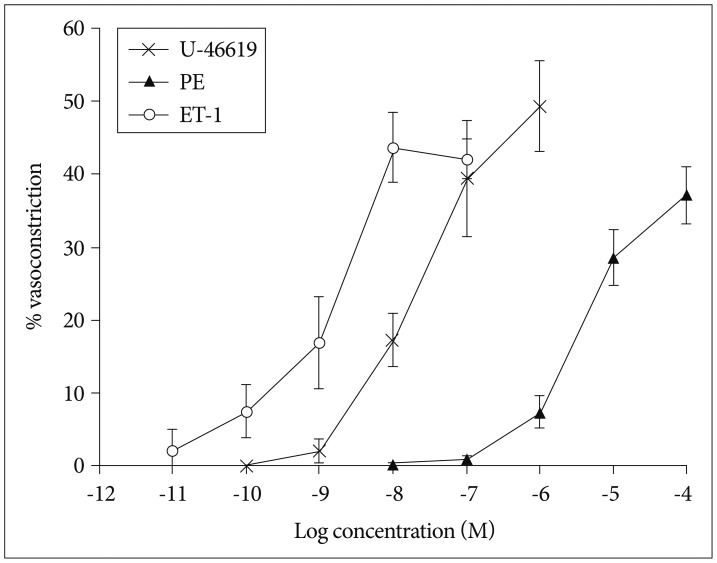
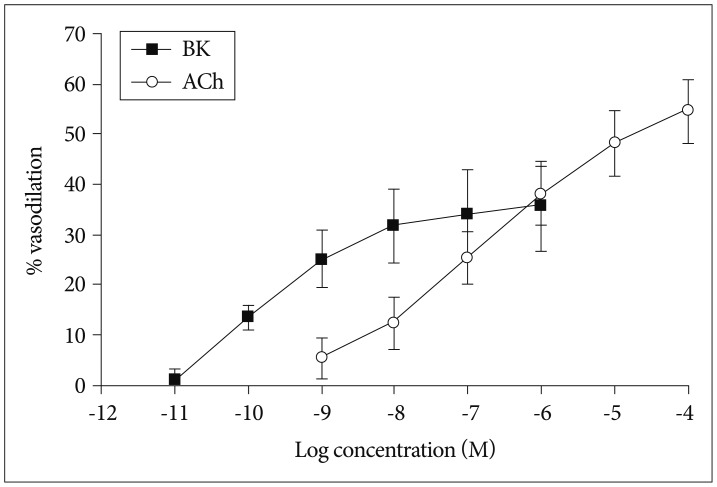
We found that the isolated mouse OAs were able to constrict in response to vasoconstrictors, including KCl, phenylephrine, endothelin-1, and prostaglandin PGH2. Moreover, this isolated vessel demonstrated vasodilation in a dose-dependent manner when vasodilatory agents, acetylcholine and bradykinin, were applied.
CONCLUSION
Our findings suggest that the isolated olfactory artery would provide as a useful ex vivo model to study the molecular and cellular mechanisms of vascular function underlying cerebrovascular disorders and the direct effects of such disease-modifying pathways on cerebrovascular function utilizing pharmacological agents and genetically modified mouse models.
Keyword
MeSH Terms
-
Animals
Arteries*
Arterioles
Bradykinin
Brain
Cerebral Arteries
Cerebrovascular Disorders
Cholinergic Agents
Connective Tissue
Endothelin-1
Mice*
Phenylephrine
Prostaglandin H2
Rats
Vasoconstriction
Vasoconstrictor Agents
Vasodilation
Bradykinin
Cholinergic Agents
Endothelin-1
Phenylephrine
Prostaglandin H2
Vasoconstrictor Agents
Figure
Reference
-
1. Bai N, Moien-Afshari F, Washio H, Min A, Laher I. Pharmacology of the mouse-isolated cerebral artery. Vascul Pharmacol. 2004; 41:97–106. PMID: 15380735.
Article2. Brown JO. The morphology of circulus arteriosus cerebri in rats. Anat Rec. 1966; 156:99–106. PMID: 5970448.
Article3. Coyne EF, Ngai AC, Meno JR, Winn HR. Methods for isolation and characterization of intracerebral arterioles in the C57/BL6 wild-type mouse. J Neurosci Methods. 2002; 120:145–153. PMID: 12385764.
Article4. Dacey RG Jr, Bassett JE. Cholinergic vasodilation of intracerebral arterioles in rats. Am J Physiol. 1987; 253(5 Pt 2):H1253–H1260. PMID: 3479909.
Article5. Dacey RG Jr, Duling BR. A study of rat intracerebral arterioles : methods, morphology, and reactivity. Am J Physiol. 1982; 243:H598–H606. PMID: 7124967.6. Dahl E. Microscopic observations on cerebral arteries. In : Cervós-Navarro J, Betz E, Matakas F, Wiillenweber R, editors. The Cerebral Vessel Wall. New York: Raven Press;1976. p. 15–21.7. Dietrich HH, Kajita Y, Dacey RG Jr. Local and conducted vasomotor responses in isolated rat cerebral arterioles. Am J Physiol. 1996; 271(3 Pt 2):H1109–H1116. PMID: 8853348.
Article8. Dorr A, Sled JG, Kabani N. Three-dimensional cerebral vasculature of the CBA mouse brain : a magnetic resonance imaging and micro computed tomography study. Neuroimage. 2007; 35:1409–1423. PMID: 17369055.
Article9. Duling BR, Gore RW, Dacey RG Jr, Damon DN. Methods for isolation, cannulation, and in vitro study of single microvessels. Am J Physiol. 1981; 241:H108–H116. PMID: 7195654.
Article10. Edvinsson L, Krause DN. The blood vessel wall. Endothelial and smooth muscle cells. In : Edvinsson L, Krause DN, editors. Cerebral Blood Flow and Metabolism. ed 2. Philadelphia: Lippincott Williams & Wilkins;2002. p. 30–42.11. Harper AM, Deshmukh VD, Rowan JO, Jennett WB. The influence of sympathetic nervous activity on cerebral blood flow. Arch Neurol. 1972; 27:1–6. PMID: 4626103.
Article12. Horiuchi T, Dietrich HH, Hongo K, Dacey RG Jr. Mechanism of extracellular K+-induced local and conducted responses in cerebral penetrating arterioles. Stroke. 2002; 33:2692–2699. PMID: 12411663.
Article13. Horiuchi T, Dietrich HH, Tsugane S, Dacey RG Jr. Role of potassium channels in regulation of brain arteriolar tone : comparison of cerebrum versus brain stem. Stroke. 2001; 32:218–224. PMID: 11136940.
Article14. Kidoguchi K, Tamaki M, Mizobe T, Koyama J, Kondoh T, Kohmura E, et al. In vivo X-ray angiography in the mouse brain using synchrotron radiation. Stroke. 2006; 37:1856–1861. PMID: 16741182.
Article15. Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL Jr. Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol. 1978; 234:H371–H383. PMID: 645875.
Article16. Morimoto M, Miyamoto S, Mizoguchi A, Kume N, Kita T, Hashimoto N. Mouse model of cerebral aneurysm : experimental induction by renal hypertension and local hemodynamic changes. Stroke. 2002; 33:1911–1915. PMID: 12105374.17. Morita M, Ohkawa M, Miyazaki S, Ishimaru T, Umetani K, Suzuki K. Simultaneous observation of superficial cortical and intracerebral microvessels in vivo during reperfusion after transient forebrain ischemia in rats using synchrotron radiation. Brain Res. 2007; 1158:116–122. PMID: 17540351.
Article18. Rosenblum WI. A review of vasomotor responses of arterioles on the surface of the mouse brain : the necessary prelude to studies using genetically manipulated mice. Microcirculation. 1998; 5:129–138. PMID: 9789254.
Article19. Sasaki T, Kassell NF, Torner JC, Maixner W, Turner DM. Pharmacological comparison of isolated monkey and dog cerebral arteries. Stroke. 1985; 16:482–489. PMID: 2860741.
Article20. Toda N. Influence of dopamine and noradrenaline on isolated cerebral arteries of the dog. Br J Pharmacol. 1976; 58:121–126. PMID: 974370.
Article21. Tong XK, Hamel E. Transforming growth factor-beta 1 impairs endothelin-1-mediated contraction of brain vessels by inducing mitogen-activated protein (MAP) kinase phosphatase-1 and inhibiting p38 MAP kinase. Mol Pharmacol. 2007; 72:1476–1483. PMID: 17848599.
Article22. Tong XK, Nicolakakis N, Kocharyan A, Hamel E. Vascular remodeling versus amyloid beta-induced oxidative stress in the cerebrovascular dysfunctions associated with Alzheimer's disease. J Neurosci. 2005; 25:11165–11174. PMID: 16319316.
Article23. Uchida E, Bohr DF, Hoobler SW. A method for studying isolated resistance vessels from rabbit mesentery and brain and their responses to drugs. Circ Res. 1967; 21:525–536. PMID: 4293658.
Article24. Villringer A, Haberl RL, Dirnagl U, Anneser F, Verst M, Einhäupl KM. Confocal laser microscopy to study microcirculation on the rat brain surface in vivo. Brain Res. 1989; 504:159–160. PMID: 2598012.
Article25. Vinall PE, Simeone FA. Cerebral autoregulation : an in vitro study. Stroke. 1981; 12:640–642. PMID: 7303050.26. Wei EP, Raper AJ, Kontos HA, Patterson JL Jr. Determinants of response of pial arteries to norepinephrine and sympathetic nerve stimulation. Stroke. 1975; 6:654–658. PMID: 1198630.
Article27. Wiland C. Comparative study on structure and variation in basal arteries of the brain in laboratory mouse. Anat Anz. 1974; 135:455–464. PMID: 4417827.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mouse Model for the Research of Sinusitis Induced Olfactory Dysfunction
- The YSK Olfactory Function Test: Development of a New Korean Olfactory Test
- Computational evaluation of interactions between olfactory receptor OR2W1 and its ligands
- The Impact of Toxicants on the Olfactory System
- A Case of Primary Olfactory Neuroblastoma of the Sphenoid Sinus