Korean J Urol.
2012 Sep;53(9):607-613. 10.4111/kju.2012.53.9.607.
Prostate-Specific Antigen Nadir and Time to Prostate-Specific Antigen Nadir Following Maximal Androgen Blockade Independently Predict Prognosis in Patients with Metastatic Prostate Cancer
- Affiliations
-
- 1Department of Urology, Ajou University School of Medicine, Suwon, Korea. sejoong@ajou.ac.kr
- 2Department of Urology, Bundang Jesaeng General Hospital, Seongnam, Korea.
- KMID: 2061698
- DOI: http://doi.org/10.4111/kju.2012.53.9.607
Abstract
- PURPOSE
To evaluate the influence of prostate-specific antigen (PSA) kinetics following maximal androgen blockade (MAB) on disease progression and cancer-specific survival in patients with metastatic, hormone-sensitive prostate cancer.
MATERIALS AND METHODS
One hundred thirty-one patients with metastatic, hormone-sensitive prostate cancer treated with MAB at our institution were included in this study. Patients' characteristics, PSA at MAB initiation, PSA nadir, time to PSA nadir (TTN), and PSA decline were analyzed by using univariate and multivariate analysis.
RESULTS
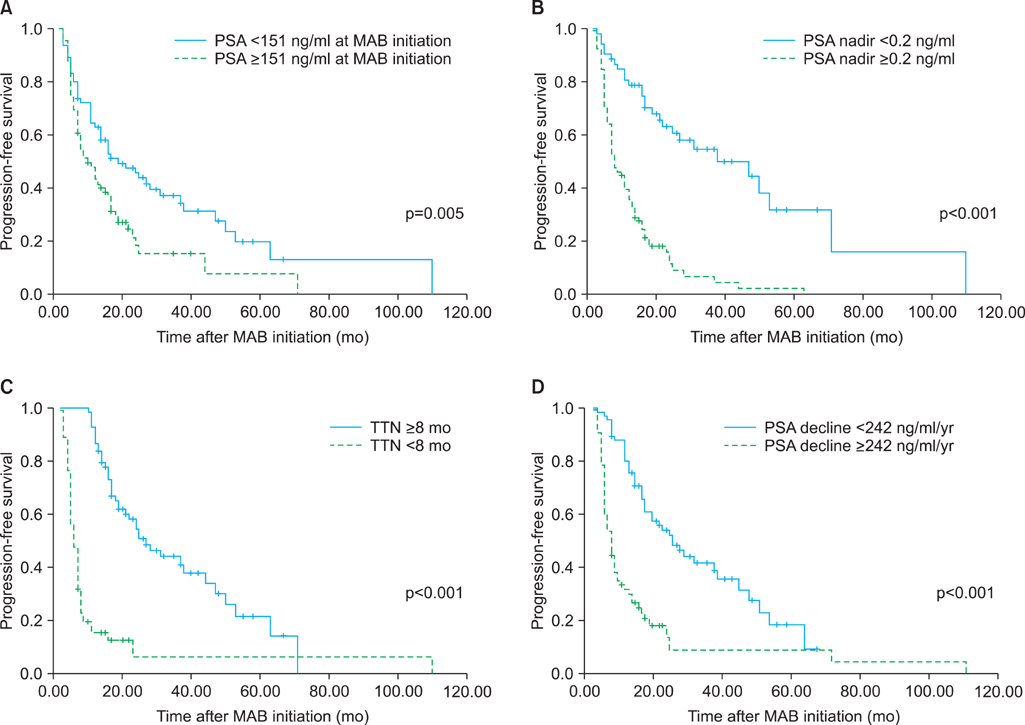
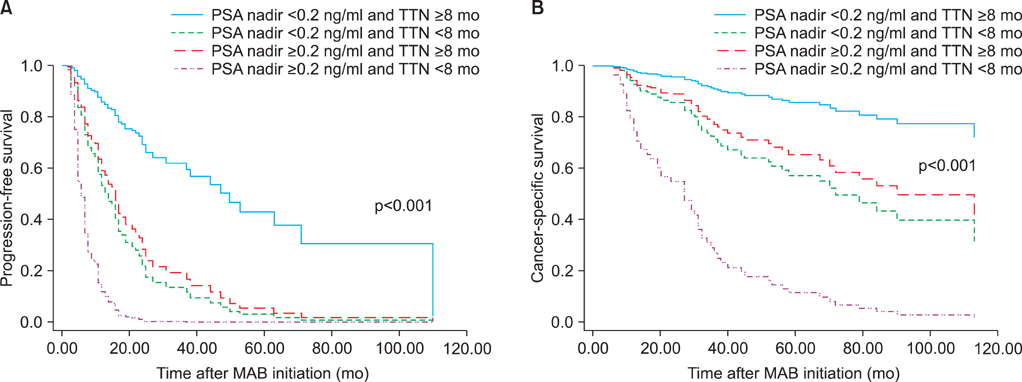
At a median follow-up of 30 months, 97 patients (74.0%) showed disease progression and 65 patients (49.6%) died. Fifty-nine patients (45.0%) died from prostate cancer. In the univariate analysis, PSA at MAB initiation, PSA nadir, TTN, and PSA decline were significant predictors of progression-free survival. Also, PSA nadir, TTN, and PSA decline were significant predictors of cancer-specific survival. In the multivariate analysis, higher PSA nadir (> or =0.2 ng/ml) and shorter TTN (<8 months) were independent predictors of shorter progression-free and cancer-specific survival. In the combined analysis of PSA nadir and TTN, patients with higher PSA nadir and shorter TTN had the worst progression-free survival (hazard ratio [HR], 14.098; p<0.001) and cancer-specific survival (HR, 14.050; p<0.001) compared with those with lower PSA nadir and longer TTN.
CONCLUSIONS
Our results suggest that higher PSA nadir level and shorter TTN following MAB are associated with higher risk of disease progression and poorer survival in patients with metastatic, hormone-sensitive prostate cancer. Furthermore, these two variables have a synergistic effect on the outcome.
MeSH Terms
Figure
Cited by 1 articles
-
Prognostic Impacts of Metastatic Site and Pain on Progression to Castrate Resistance and Mortality in Patients with Metastatic Prostate Cancer
Kyo Chul Koo, Sang Un Park, Ki Hong Kim, Koon Ho Rha, Sung Joon Hong, Seung Choul Yang, Byung Ha Chung
Yonsei Med J. 2015;56(5):1206-1212. doi: 10.3349/ymj.2015.56.5.1206.
Reference
-
1. Kim SJ, Kim SI. Incidence, epidemiology and patterns of progression of prostate cancer. J Korean Med Assoc. 2010. 53:92–97.2. Hori S, Jabbar T, Kachroo N, Vasconcelos JC, Robson CN, Gnanapragasam VJ. Outcomes and predictive factors for biochemical relapse following primary androgen deprivation therapy in men with bone scan negative prostate cancer. J Cancer Res Clin Oncol. 2011. 137:235–241.3. Arai Y, Yoshiki T, Yoshida O. Prognostic significance of prostate specific antigen in endocrine treatment for prostatic cancer. J Urol. 1990. 144:1415–1419.4. Cooper EH, Armitage TG, Robinson MR, Newling DW, Richards BR, Smith PH, et al. Prostatic specific antigen and the prediction of prognosis in metastatic prostatic cancer. Cancer. 1990. 66:5 Suppl. 1025–1028.5. Mulders PF, Fernandez del Moral P, Theeuwes AG, Oosterhof GO, van Berkel HT, Debruyne FM. Value of biochemical markers in the management of disseminated prostatic cancer. Eur Urol. 1992. 21:2–5.6. D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998. 280:969–974.7. Zhou P, Chen MH, McLeod D, Carroll PR, Moul JW, D'Amico AV. Predictors of prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Clin Oncol. 2005. 23:6992–6998.8. May M, Gunia S, Helke C, Braun KP, Pickenhain S, Hoschke B. Is it possible to provide a prognosis after radical prostatectomy for prostate cancer by means of a PSA regression model? Int J Biol Markers. 2005. 20:112–118.9. King CR, Presti JC, Brooks JD, Gill H, Spiotto MT. Postoperative prostate-specific antigen velocity independently predicts for failure of salvage radiotherapy after prostatectomy. Int J Radiat Oncol Biol Phys. 2008. 70:1472–1477.10. Ercole CJ, Lange PH, Mathisen M, Chiou RK, Reddy PK, Vessella RL. Prostatic specific antigen and prostatic acid phosphatase in the monitoring and staging of patients with prostatic cancer. J Urol. 1987. 138:1181–1184.11. Miller JI, Ahmann FR, Drach GW, Emerson SS, Bottaccini MR. The clinical usefulness of serum prostate specific antigen after hormonal therapy of metastatic prostate cancer. J Urol. 1992. 147(3 Pt 2):956–961.12. Park YH, Hwang IS, Jeong CW, Kim HH, Lee SE, Kwak C. Prostate specific antigen half-time and prostate specific antigen doubling time as predictors of response to androgen deprivation therapy for metastatic prostate cancer. J Urol. 2009. 181:2520–2524.13. Kwak C, Jeong SJ, Park MS, Lee E, Lee SE. Prognostic significance of the nadir prostate specific antigen level after hormone therapy for prostate cancer. J Urol. 2002. 168:995–1000.14. Morote J, Esquena S, Abascal JM, Trilla E, Cecchini L, Raventos CX, et al. Usefulness of prostate-specific antigen nadir as predictor of androgen-independent progression of metastatic prostate cancer. Int J Biol Markers. 2005. 20:209–216.15. Morote J, Trilla E, Esquena S, Abascal JM, Reventos J. Nadir prostate-specific antigen best predicts the progression to androgen-independent prostate cancer. Int J Cancer. 2004. 108:877–881.16. Benaim EA, Pace CM, Lam PM, Roehrborn CG. Nadir prostate-specific antigen as a predictor of progression to androgen-independent prostate cancer. Urology. 2002. 59:73–78.17. Huang SP, Bao BY, Wu MT, Choueiri TK, Goggins WB, Huang CY, et al. Impact of prostate-specific antigen (PSA) nadir and time to PSA nadir on disease progression in prostate cancer treated with androgen-deprivation therapy. Prostate. 2011. 71:1189–1197.18. Stamey TA, Kabalin JN, Ferrari M, Yang N. Prostate specific antigen in the diagnosis and treatment of adenocarcinoma of the prostate. IV. Anti-androgen treated patients. J Urol. 1989. 141:1088–1090.19. Park BJ, Lee YG, Ahn HK. Prognostic significance of prostate-specific antigen level two months after maximal androgen blockade in metastatic prostate cancer. Korean J Urol. 2003. 44:855–860.20. Petros JA, Andriole GL. Serum PSA after antiandrogen therapy. Urol Clin North Am. 1993. 20:749–756.21. Furuya Y, Akimoto S, Akakura K, Igarashi T, Murakami S, Shimazaki J, et al. Response of prostate-specific antigen after androgen withdrawal and prognosis in men with metastatic prostate cancer. Urol Int. 1998. 60:28–32.22. Park SC, Choi HY, Kim CS, Hong SJ, Kim WJ, Lee SE, et al. Predictive variables of the progression to androgen independent prostate cancer after combined androgen blockade. Korean J Urol. 2007. 48:408–415.23. Choueiri TK, Xie W, D'Amico AV, Ross RW, Hu JC, Pomerantz M, et al. Time to prostate-specific antigen nadir independently predicts overall survival in patients who have metastatic hormone-sensitive prostate cancer treated with androgen-deprivation therapy. Cancer. 2009. 115:981–987.24. Huang SP, Bao BY, Wu MT, Choueiri TK, Goggins WB, Liu CC, et al. Significant associations of prostate-specific antigen nadir and time to prostate-specific antigen nadir with survival in prostate cancer patients treated with androgen-deprivation therapy. Aging Male. 2012. 15:34–41.25. Sasaki T, Onishi T, Hoshina A. Nadir PSA level and time to PSA nadir following primary androgen deprivation therapy are the early survival predictors for prostate cancer patients with bone metastasis. Prostate Cancer Prostatic Dis. 2011. 14:248–252.26. Hussain M, Tangen CM, Higano C, Schelhammer PF, Faulkner J, Crawford ED, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162). J Clin Oncol. 2006. 24:3984–3990.27. Stewart AJ, Scher HI, Chen MH, McLeod DG, Carroll PR, Moul JW, et al. Prostate-specific antigen nadir and cancer-specific mortality following hormonal therapy for prostate-specific antigen failure. J Clin Oncol. 2005. 23:6556–6560.28. Ross RW, Xie W, Regan MM, Pomerantz M, Nakabayashi M, Daskivich TJ, et al. Efficacy of androgen deprivation therapy (ADT) in patients with advanced prostate cancer: association between Gleason score, prostate-specific antigen level, and prior ADT exposure with duration of ADT effect. Cancer. 2008. 112:1247–1253.29. Lee SY, Kim YS, Hong SJ. Clinical response of combined androgen blockade in metastatic prostate cancers. Korean J Urol. 2000. 41:361–366.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intermittent Androgen Deprivation with Goserelin and Flutamide for Prostate Cancer: a Pilot Study
- Clinical Significance of Free-to-Total Prostate-Specific Antigen (PSA) Ratio in Advanced Prostate Cancer Patients with PSA Less than 0.1 ng/ml after Hormone Treatment
- Prognostic Significance of the Nadir Prostate Specific Antigen Level after Hormone Therapy for Prostate Cancer Patients
- Intermittent Androgen Deprivation(IAD) with Cyproterone Acetate Monotherapy for Prostate Cancer: A preliminary report
- Change of serum prostate specific antigen values after radiation therapy in prostate cancer