J Korean Acad Prosthodont.
2015 Jan;53(1):9-18. 10.4047/jkap.2015.53.1.9.
The effect of blasting and anodizing-combined treatment of implant surface on response of osteoblast-like cell
- Affiliations
-
- 1Department of Prosthodontics, School of Dentistry, Pusan National University, Yangsan, Republic of Korea. huhjb@pusan.ac.kr
- 2Department of Oral Anatomy, School of Dentistry, Pusan National University, Yangsan, Republic of Korea.
- KMID: 2057202
- DOI: http://doi.org/10.4047/jkap.2015.53.1.9
Abstract
- PURPOSE
The purpose of this study is to examine characteristics of implant surface with RBM and anodizing treatments, and to evaluate the responses of osteoblast-like cell (MG-63 cell).
MATERIALS AND METHODS
Grade IV titanium disks were fabricated (Diameter 10 mm, thickness 3 mm). Anodizing treatment (ASD) group, RBM and anodizing treatment (RBM/ASD) group, control (machined surface) group were divided. In this study, osteoblast-like cell was used for experiments. The experiments consist of surface characteristics evaluation by FE-SEM images, energy dispersive spectroscopy and stereo-SEM. In order to evaluate cell adhesion evaluation by crystal violet assay and observe cells form by confocal laser microscopy. To assess cell proliferation by XTT assay, cell differentiation by RT-PCR and mineralization by Alizarin red S stain assay. ELISA analyzer was used for Quantitative evaluation. Comparative analysis was run by one-way ANOVA (SPSS version 18.0). Differences were considered statistically significant at P<.05.
RESULTS
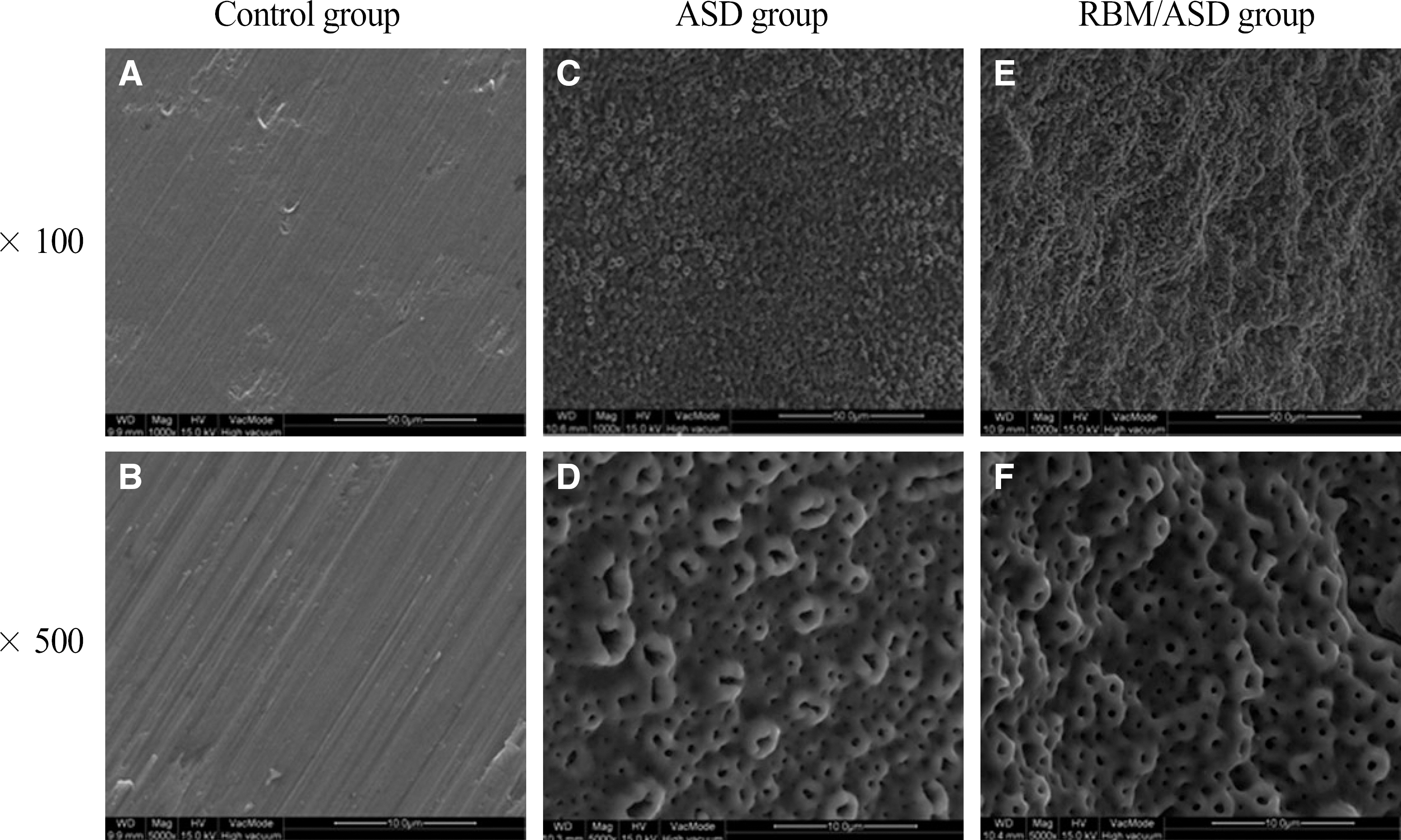
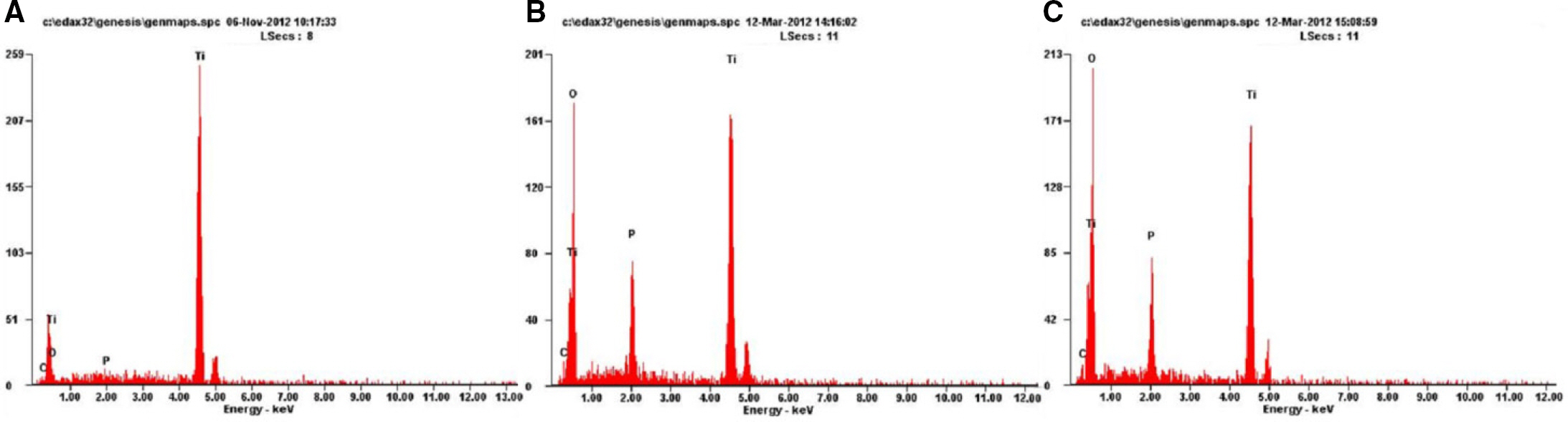
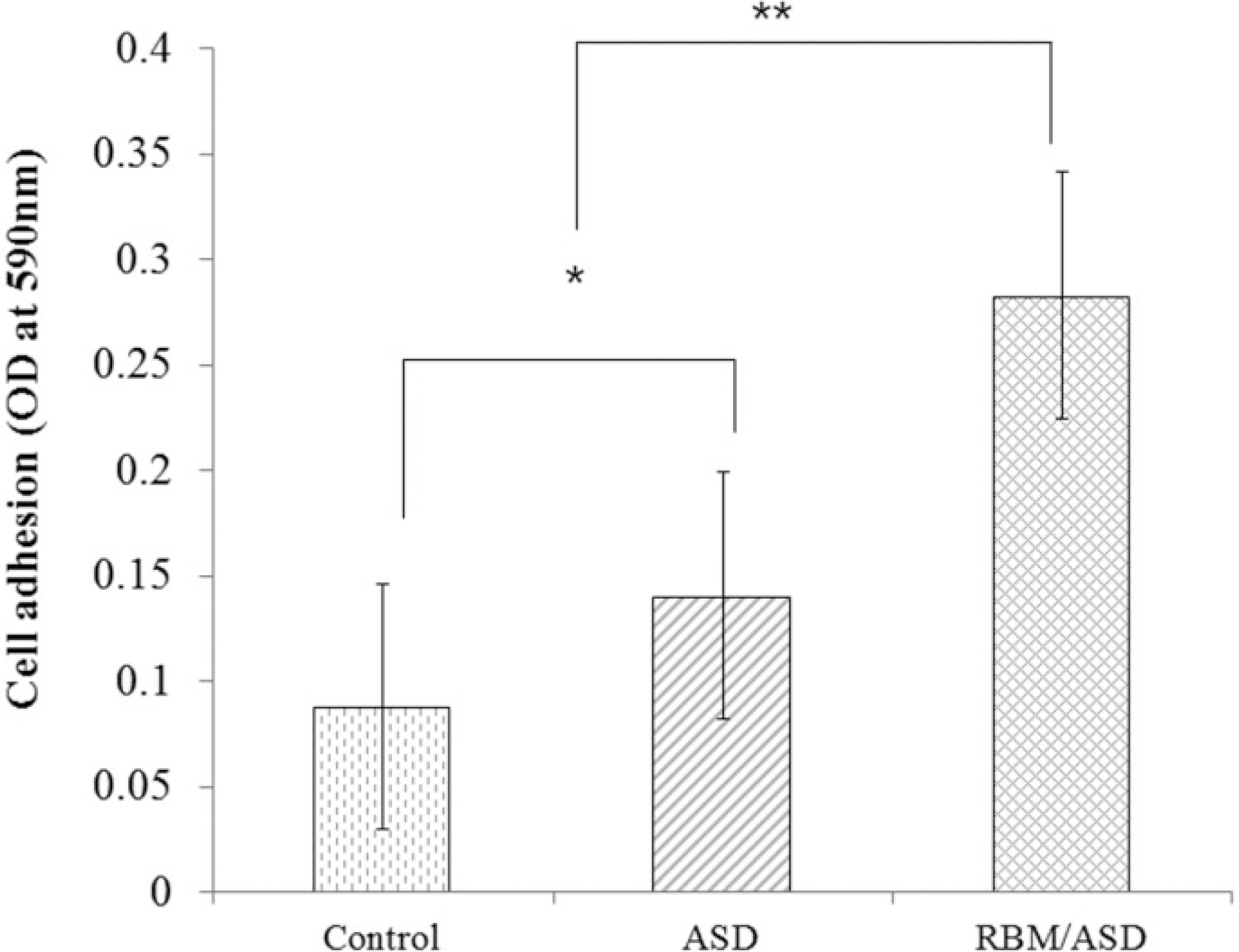
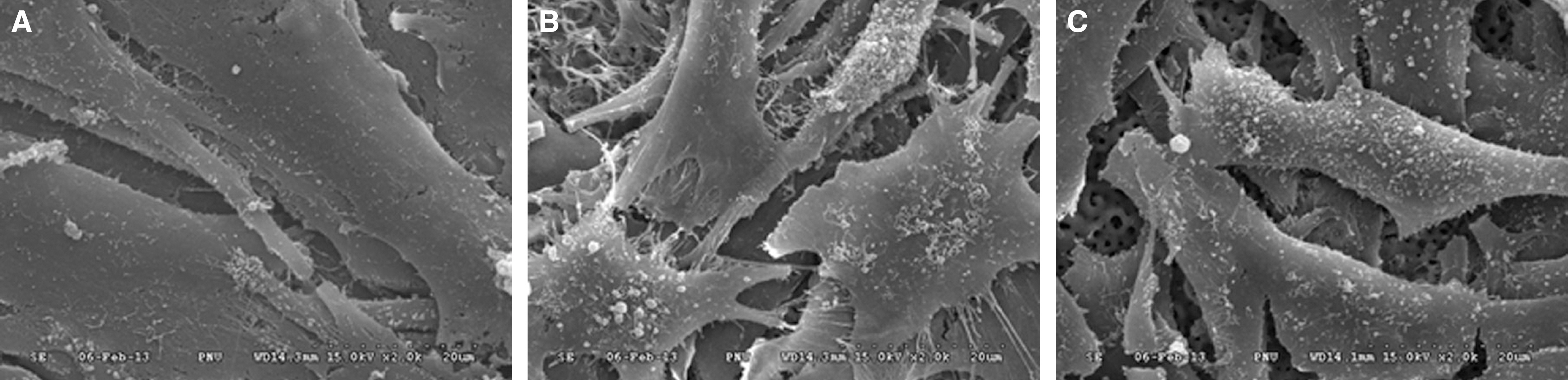
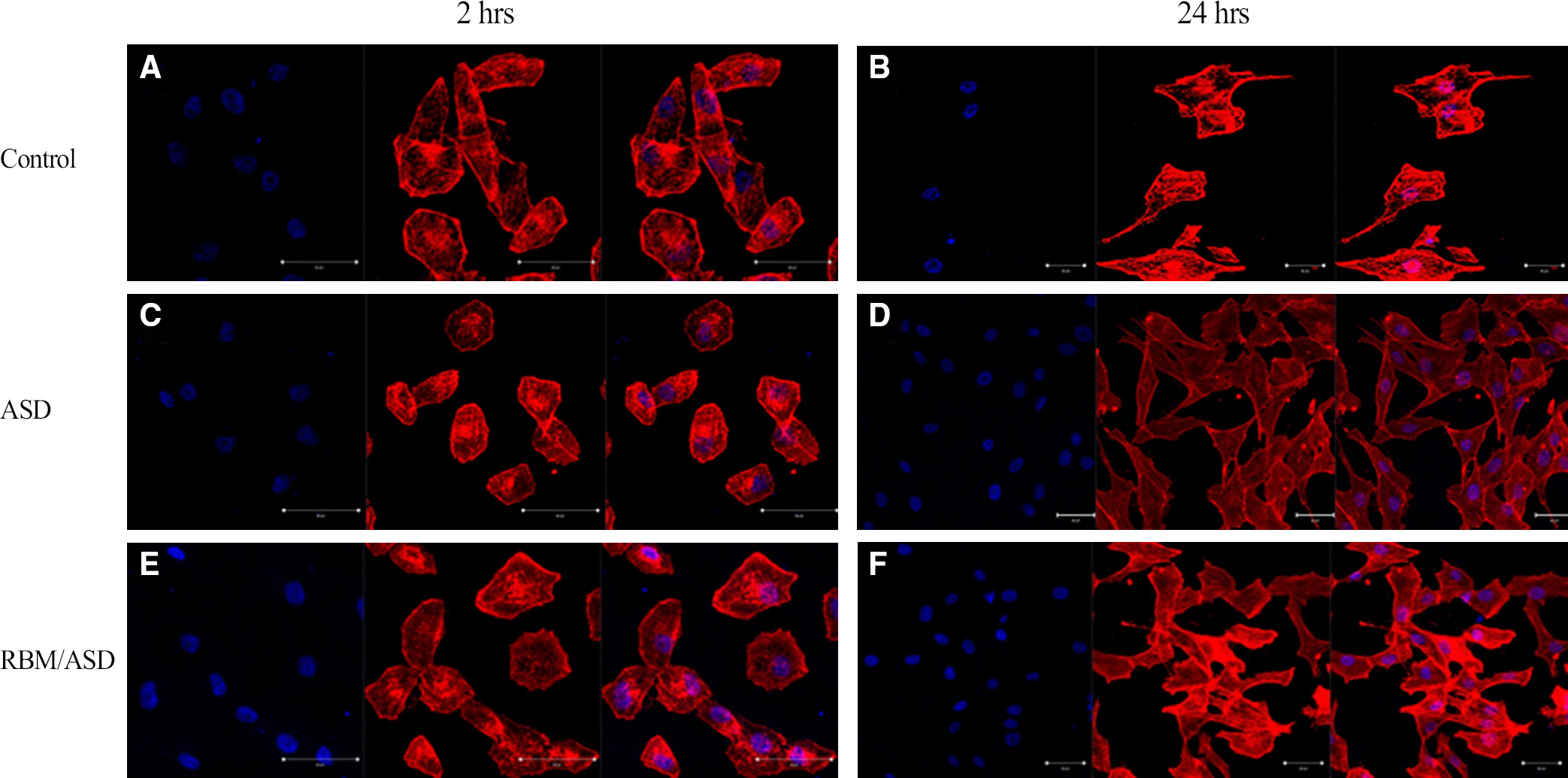
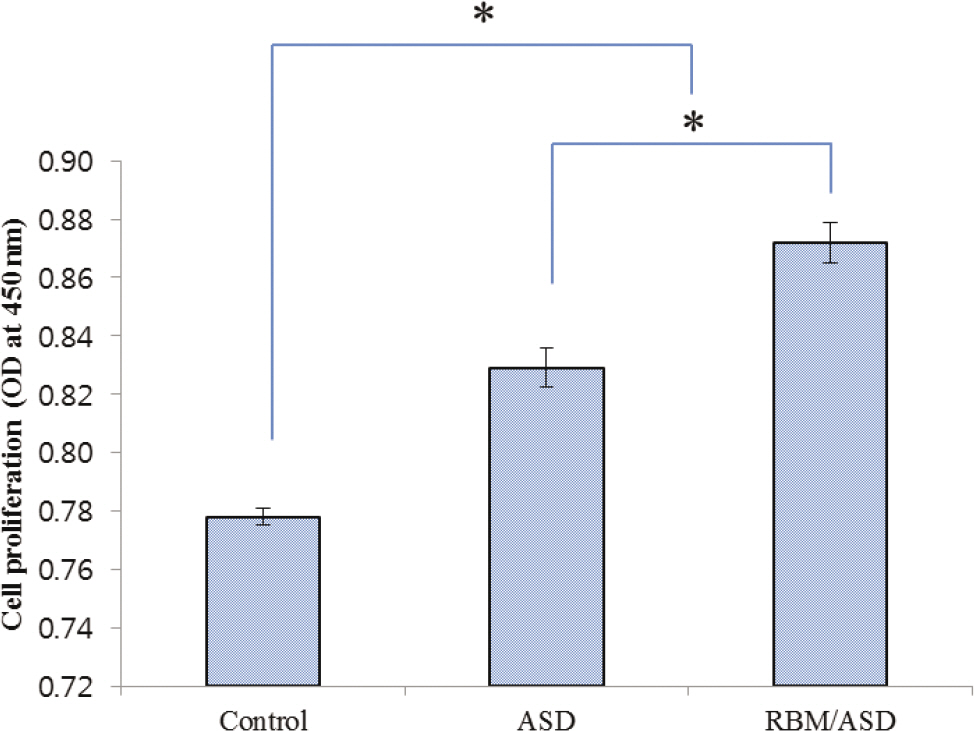
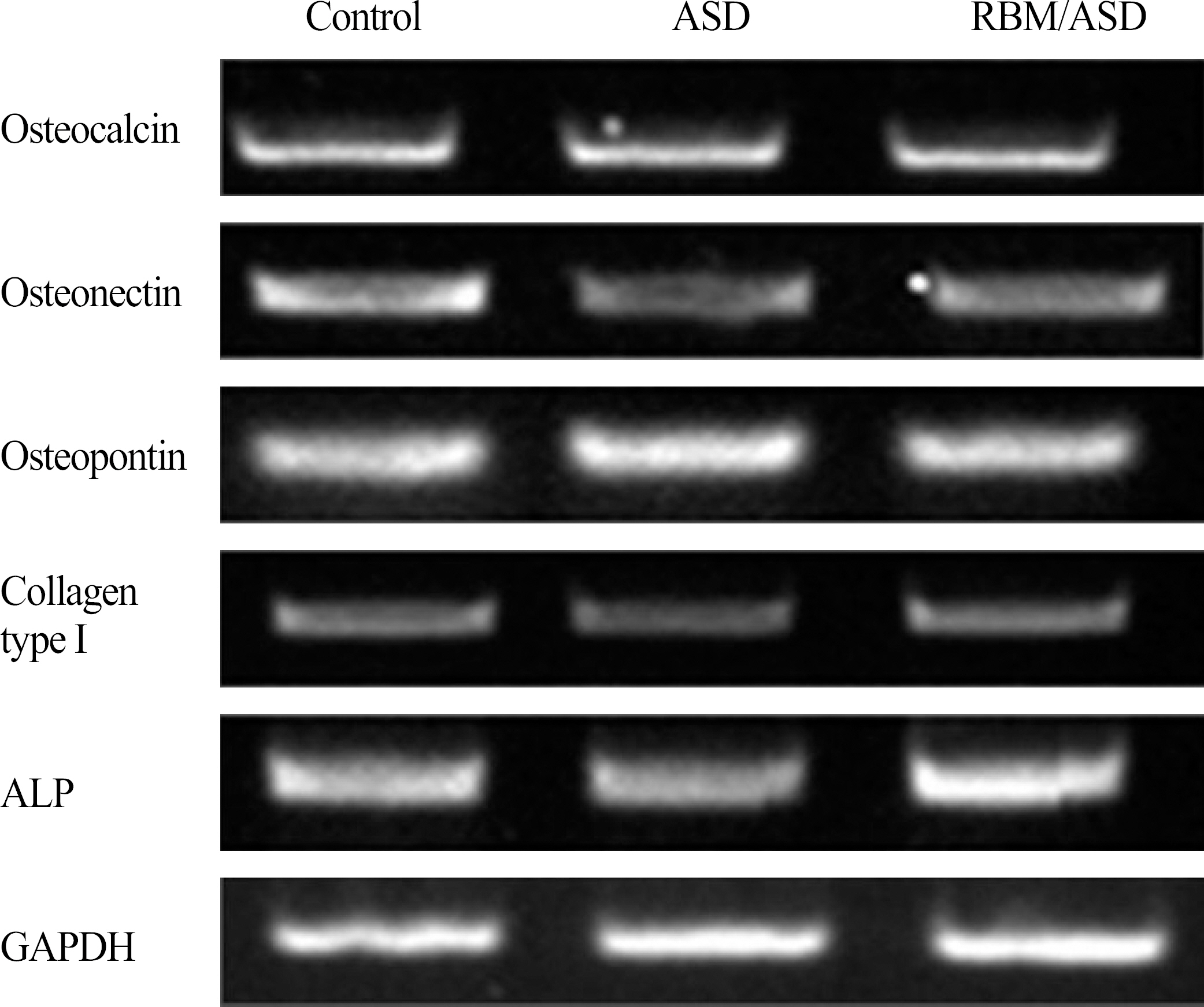
In ASD group and RBM/ASD group, the surface shape of the crater was observed and components of oxygen and phosphate ions in comparison with the control group were detected. The surface average roughness was obtained 0.08 +/- 0.04 microm in the control group, 0.52 +/- 0.14 microm in ASD group and 1.45 +/- 0.25 microm in RBM/ASD group. In cell response experiments, ASD group and RBM/ASD group were significantly higher values than control group in cell adhesion and mineralization phase, control group was the highest values in the proliferative phase. In RT-PCR experiments, RBM/ASD group was showed higher ALP activity than other groups. RBM/ASD group in comparison with ASD group was significantly higher value for cell adhesion and proliferation phase.
CONCLUSION
In the limitation of this study, we are concluded that the surface treatment with RBM/ASD seems more effective than ASD alone or machined surface on cellular response.
Keyword
MeSH Terms
Figure
Reference
-
1. Bra�nemark PI, Adell R, Breine U, Hansson BO, Lindstro¨m J, Ohlsson A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969; 3:81–100.2. Schroeder A, van der Zypen E, Stich H, Sutter F. The reactions of bone, connective tissue, and epithelium to endosteal implants with titanium-sprayed surfaces. J Maxillofac Surg. 1981; 9:15–25.
Article3. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and pro-posed criteria of success. Int J Oral Maxillofac Implants. 1986; 1:11–25.4. Le Gue′hennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007; 23:844–54.5. Buser D, Schenk RK, Steinemann S, Fiorellini JP, Fox CH, Stich H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991; 25:889–902.6. Albrektsson T, Bra�nemark PI, Hansson HA, Lindstro¨m J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 1981; 52:155–70.7. Cochran DL, Schenk RK, Lussi A, Higginbottom FL, Buser D. Bone response to unloaded and loaded titanium implants with a sandblasted and acid-etched surface: a histometric study in the canine mandible. J Biomed Mater Res. 1998; 40:1–11.
Article8. Roberts WE, Garetto LP, DeCastro RA. Remodeling of devitalized bone threatens periosteal margin integrity of endosseous titanium implants with threaded or smooth surfaces: indications for provisional loading and axially directed occlusion. J Indiana Dent Assoc. 1989; 68:19–24.9. Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL, Hoffmann B, Lussi A, Steinemann SG. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res. 2004; 83:529–33.
Article10. He FM, Yang GL, Li YN, Wang XX, Zhao SF. Early bone response to sandblasted, dual acid-etched and H2O2/HCl treated titanium implants: an experimental study in the rabbit. Int J Oral Maxillofac Surg. 2009; 38:677–81.
Article11. Yang GL, He FM, Yang XF, Wang XX, Zhao SF. Bone responses to titanium implants surface-roughened by sandblasted and double etched treatments in a rabbit model. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 106:516–24.
Article12. Aparicio C, Gil FJ, Fonseca C, Barbosa M, Planell JA. Corrosion behaviour of commercially pure titanium shot blasted with different materials and sizes of shot particles for dental implant ap-plications. Biomaterials. 2003; 24:263–73.
Article13. Piattelli M, Scarano A, Paolantonio M, Iezzi G, Petrone G, Piattelli A. Bone response to machined and resorbable blast material titanium implants: an experimental study in rabbits. J Oral Implantol. 2002; 28:2–8.
Article14. Sul YT, Johansson CB, Petronis S, Krozer A, Jeong Y, Wennerberg A, Albrektsson T. Characteristics of the surface oxides on turned and electrochemically oxidized pure titanium implants up to dielectric breakdown: the oxide thickness, micropore con-figurations, surface roughness, crystal structure and chemical composition. Biomaterials. 2002; 23:491–501.15. Lausmaa J. Surface spectroscopic characterization of titanium implant materials. J Electron Spectrosc Relat Phenom. 1996; 81:343–61.
Article16. Olefjord I, Hansson S. Surface analysis of four dental implant systems. Int J Oral Maxillofac Implants. 1993; 8:32–40.17. Machnee CH, Wagner WC, Jaarda MJ, Lang BR. Identification of oxide layers of commercially pure titanium in response to cleaning procedures. Int J Oral Maxillofac Implants. 1993; 8:529–33.18. Albrektsson T, Wennerberg A. Oral implant surfaces: Part 1-review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004; 17:536–43.19. Albrektsson T, Wennerberg A. Oral implant surfaces: Part 2-review focusing on clinical knowledge of different surfaces. Int J Prosthodont. 2004; 17:544–64.20. Stadlinger B, Lode AT, Eckelt U, Range U, Schlottig F, Hefti T, Mai R. Surface-conditioned dental implants: an animal study on bone formation. J Clin Periodontol. 2009; 36:882–91.
Article21. Lee HJ, Song KY, Yoon TH. Effect of different surface treatments to increase biocompatibility of dental implant. J Korean Acad Prosthodont. 2006; 44:594–605.22. Martin JY, Dean DD, Cochran DL, Simpson J, Boyan BD, Schwartz Z. Proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63) cultured on previously used titanium surfaces. Clin Oral Implants Res. 1996; 7:27–37.
Article23. Zhu X, Ong JL, Kim S, Kim K. Surface characteristics and structure of anodic oxide films containing Ca and P on a titanium implant material. J Biomed Mater Res. 2002; 60:333–8.
Article24. Macak JM, Tsuchiya H, Taveira L, Ghicov A, Schmuki P. Self-organized nanotubular oxide layers on Ti-6Al-7Nb and Ti-6Al-4V formed by anodization in NH4F solutions. J Biomed Mater Res A. 2005; 75:928–33.
Article25. Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992; 69:11–25.
Article26. Villarreal DR, Sogal A, Ong JL. Protein adsorption and osteoblast responses to different calcium phosphate surfaces. J Oral Implantol. 1998; 24:67–73.
Article27. Martin JY, Schwartz Z, Hummert TW, Schraub DM, Simpson J, Lankford J Jr, Dean DD, Cochran DL, Boyan BD. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J Biomed Mater Res. 1995; 29:389–401.
Article28. Wong M, Eulenberger J, Schenk R, Hunziker E. Effect of surface topology on the osseointegration of implant materials in trabecular bone. J Biomed Mater Res. 1995; 29:1567–75.
Article29. Wennerberg A. The importance of surface roughness for implant incorporation. Int J Mach Tools Manufact. 1998; 38:657–62.
Article30. Lincks J, Boyan BD, Blanchard CR, Lohmann CH, Liu Y, Cochran DL, Dean DD, Schwartz Z. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials. 1998; 19:2219–32.
Article31. Kim HJ, Son MK, Park JI, Chung HJ, Kim YJ. Biological response of primary rat calvarial cell by surface treatment of Ti-8Ta-8Nb alloy. J Korean Acad Periodontol. 2008; 38:595–602.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of implant surface treated by anodizing on proliferation of the rat osteoblast
- A study on the biological characteristics of modified titanium surface
- The effect of Er:YAG laser irradiation on the surface microstructure and roughness of TiO2 implant
- Effect of implant surface microtopography by hydroxyapatite grit-blasting on adhesion, proliferation, and differentiation of osteoblast-like cell line, MG-63
- A Comparison of the Biocompatibility of Titanium-Base Alloy According to Surface Treatments