J Gynecol Oncol.
2009 Mar;20(1):22-27. 10.3802/jgo.2009.20.1.22.
Comparative study of neoadjuvant chemotherapy before radical hysterectomy and radical surgery alone in stage IB2-IIA bulky cervical cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea. jhnam@amc.seoul.kr
- KMID: 2055644
- DOI: http://doi.org/10.3802/jgo.2009.20.1.22
Abstract
OBJECTIVE
To compare the efficacy of neoadjuvant chemotherapy with paclitaxel plus platinum followed by radical hysterectomy with radical surgery alone in patients with stage IB2-IIA bulky cervical cancer.
METHODS
From November 1999 to September 2007, stage IB2-IIA cervical cancers with tumor diameter >4 cm, as measured by MRI, were managed with two cycles of preoperative paclitaxel and platinum. As a control group, we selected 35 patients treated with radical surgery alone.
RESULTS
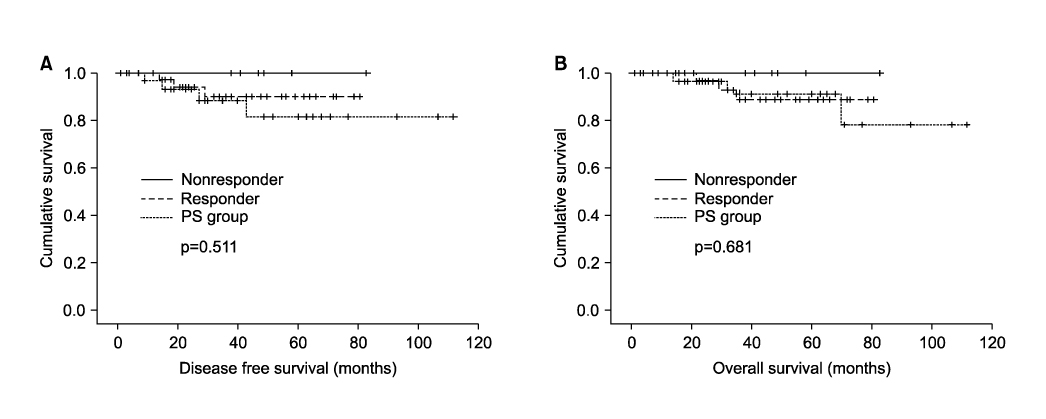
There were no significant between group differences in age, tumor size, FIGO stage, level of SCC Ag, histopathologic type and grade. Operating time, estimated blood loss, the number of lymph nodes yielded and the rate of complications were similar in the two groups. In surgical specimens, lymph-vascular space invasion (LVSI), nodal metastasis and parametrial involvement did not differ significantly between the two groups. In the neoadjuvant group, pathologic tumor size was significantly smaller and fewer patients had deep cervical invasion. Radiotherapy, alone and in the form of concurrent chemoradiation, was administered to more patients treated with radical surgery alone (82.9% vs. 52.9%, p=0.006). No recurrence was observed in patients who could avoid adjuvant radiotherapy owing to improved risk factors after neoadjuvant chemotherapy. There were no significant differences in 5-year disease free and overall survival.
CONCLUSION
As neoadjuvant chemotherapy would improve pathologic prognostic factors, adjuvant radiotherapy can be avoided, without worsening the prognosis, in patients with locally advanced bulky cervical cancer. Neoadjuvant chemotherapy would be improving the quality of life after radical hysterectomy in patients with bulky cervical cancer.
MeSH Terms
Figure
Reference
-
1. Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999. 80:827–841.2. Alvarez RD, Soong SJ, Kinney WK, Reid GC, Schray MF, Podratz KC, et al. Identification of prognostic factors and risk groups in patients found to have nodal metastasis at the time of radical hysterectomy for early-stage squamous carcinoma of the cervix. Gynecol Oncol. 1989. 35:130–135.3. Perez CA, Grigsby PW, Nene SM, Camel HM, Galakatos A, Kao MS, et al. Effect of tumor size on the prognosis of carcinoma of the uterine cervix treated with irradiation alone. Cancer. 1992. 69:2796–2806.4. Thoms WW Jr, Eifel PJ, Smith TL, Morris M, Delclos L, Wharton JT, et al. Bulky endocervical carcinoma: a 23-year experience. Int J Radiat Oncol Biol Phys. 1992. 23:491–499.5. Kim DS, Moon H, Kim KT, Hwang YY, Cho SH, Kim SR. Two-year survival: preoperative adjuvant chemotherapy in the treatment of cervical cancer stages Ib and II with bulky tumor. Gynecol Oncol. 1989. 33:225–230.6. Friedlander ML, Atkinson K, Coppleson JV, Elliot P, Green D, Houghton R, et al. The integration of chemotherapy into the management of locally advanced cervical cancer: a pilot study. Gynecol Oncol. 1984. 19:1–7.7. Sardi J, Sananes C, Giaroli A, Bayo J, Rueda NG, Vighi S, et al. Results of a prospective randomized trial with neoadjuvant chemotherapy in stage IB, bulky, squamous carcinoma of the cervix. Gynecol Oncol. 1993. 49:156–165.8. Hwang YY, Moon H, Cho SH, Kim KT, Moon YJ, Kim SR, et al. Ten-year survival of patients with locally advanced, stage ib-iib cervical cancer after neoadjuvant chemotherapy and radical hysterectomy. Gynecol Oncol. 2001. 82:88–93.9. Scarabelli C, Zarrelli A, Gallo A, Visentin MC. Multimodal treatment with neoadjuvant intraarterial chemotherapy and radical surgery in patients with stage IIIB-IVA cervical cancer: a preliminary study. Cancer. 1995. 76:1019–1026.10. Park SY, Kim BG, Kim JH, Lee JH, Lee ED, Lee KH, et al. Phase I/II study of neoadjuvant intraarterial chemotherapy with mitomycin-C, vincristine, and cisplatin in patients with stage IIb bulky cervical carcinoma. Cancer. 1995. 76:814–823.11. Giaroli A, Sananes C, Sardi JE, Maya AG, Bastardas ML, Snaidas L, et al. Lymph node metastases in carcinoma of the cervix uteri: Response to neoadjuvant chemotherapy and its impact on survival. Gynecol Oncol. 1990. 39:34–39.12. Yamakawa Y, Fujimura M, Hidaka T, Hori S, Saito S. Neoadjuvant intraarterial infusion chemotherapy in patients with stage IB2-IIIB cervical cancer. Gynecol Oncol. 2000. 77:264–270.13. Benedetti-Panici P, Greggi S, Colombo A, Amoroso M, Smaniotto D, Giannarelli D, et al. Neoadjuvant chemotherapy and radical surgery versus exclusive radiotherapy in locally advanced squamous cell cervical cancer: results from the Italian multicenter randomized study. J Clin Oncol. 2002. 20:179–188.14. Neoadjuvant Chemotherapy for Locally Advanced Cervical Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy for locally advanced cervical cancer: a systematic review and meta-analysis of individual patient data from 21 randomised trials. Eur J Cancer. 2003. 39:2470–2486.15. Eddy GL, Bundy BN, Creasman WT, Spirtos NM, Mannel RS, Hannigan E, et al. Treatment of ("bulky") stage IB cervical cancer with or without neoadjuvant vincristine and cisplatin prior to radical hysterectomy and pelvic/para-aortic lymphadenectomy: A phase III trial of the gynecologic oncology group. Gynecol Oncol. 2007. 106:362–369.16. Kim DS, Moon H, Hwang YY, Cho SH. Preoperative adjuvant chemotherapy in the treatment of cervical cancer stage Ib, IIa, and IIb with bulky tumor. Gynecol Oncol. 1988. 29:321–332.17. Weiner SA, Aristizabal S, Alberts DS, Surwit EA, Deatherage-Deuser K. A phase II trial of mitomycin, vincristine, bleomycin, and cisplatin (MOBP) as neoadjuvant therapy in high-risk cervical carcinoma. Gynecol Oncol. 1988. 30:1–6.18. Behtash N, Nazari Z, Ayatollahi H, Modarres M, Ghaemmaghami F, Mousavi A. Neoadjuvant chemotherapy and radical surgery compared to radical surgery alone in bulky stage IB-IIA cervical cancer. Eur J Surg Oncol. 2006. 32:1226–1230.19. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000. 92:205–216.20. Piver MS, Chung WS. Prognostic significance of cervical lesion size and pelvic node metastases in cervical carcinoma. Obstet Gynecol. 1975. 46:507–510.21. Fuller AF, Elliott N, Kosloff C, Lewis JL. Lymph node metastases from carcinoma of the cervix, stages IB and IIA: implications for prognosis and treatment. Gynecologic Oncology. 1982. 13:165–174.22. Mohar A, Frias-Mendivil M. Epidemiology of cervical cancer. Cancer Invest. 2000. 18:584–590.23. Delgado G, Bundy BN, Fowler WC Jr, Stehman FB, Sevin B, Creasman WT, et al. A prospective surgical pathological study of stage I squamous carcinoma of the cervix: a Gynecologic Oncology Group Study. Gynecol Oncol. 1989. 35:314–320.24. Modarress M, Maghami FQ, Golnavaz M, Behtash N, Mousavi A, Khalili GR. Comparative study of chemoradiation and neoadjuvant chemotherapy effects before radical hysterectomy in stage IB-IIB bulky cervical cancer and with tumor diameter greater than 4 cm. Int J Gynecol Cancer. 2005. 15:483–488.25. Kigawa J, Minagawa Y, Ishihara H, Itamochi H, Kanamori Y, Terakawa N. The role of neoadjuvant intraarterial infusion chemotherapy with cisplatin and bleomycin for locally advanced cervical cancer. Am J Clin Oncol. 1996. 19:255–259.26. Namkoong SE, Park JS, Kim JW, Bae SN, Han GT, Lee JM, et al. Comparative study of the patients with locally advanced stages I and II cervical cancer treated by radical surgery with and without preoperative adjuvant chemotherapy. Gynecol Oncol. 1995. 59:136–142.27. Paladini D, Raspagliesi F, Fontanelli R, Ntousias V. Radical surgery after induction chemotherapy in locally advanced cervical cancer: a feasibility study. Int J Gynecol Cancer. 1995. 5:296–300.28. Panici PB, Scambia G, Baiocchi G, Greggi S, Ragusa G, Gallo A, et al. Neoadjuvant chemotherapy and radical surgery in locally advanced cervical cancer: prognostic factors for response and survival. Cancer. 1991. 67:372–379.29. Eddy GL, Manetta A, Alvarez RD, Williams L, Creasman WT. Neoadjuvant chemotherapy with vincristine and cisplatin followed by radical hysterectomy and pelvic lymphadenectomy for FIGO stage IB bulky cervical cancer: a Gynecologic Oncology Group pilot study. Gynecol Oncol. 1995. 57:412–416.30. Huang HJ, Chang TC, Hong JH, Tseng CJ, Chou HH, Huang KG, et al. Prognostic value of age and histologic type in neoadjuvant chemotherapy plus radical surgery for bulky (>/=4 cm) stage IB and IIA cervical carcinoma. Int J Gynecol Cancer. 2003. 13:204–211.31. Duenas-Gonzalez A, Lopez-Graniel C, Gonzalez-Enciso A, Cetina L, Rivera L, Mariscal I, et al. A phase II study of multi-modality treatment for locally advanced cervical cancer: neoadjuvant carboplatin and paclitaxel followed by radical hysterectomy and adjuvant cisplatin chemoradiation. Ann Oncol. 2003. 14:1278–1284.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Management of FIGO stage IB2 Cervical Cancer
- The twenty-first century role of Piver-Rutledge type III radical hysterectomy and FIGO stage IA, IB1, and IB2 cervical cancer in the era of robotic surgery: a personal perspective
- Neoadjuvant Chemotherapy for the Bulky-endophytic or Barrel-shaped Cervix
- Neoadjuvant chemotherapy in stage IB2 cervical cancer
- Vaginal evisceration after radical hysterectomy and adjuvant radiation