J Periodontal Implant Sci.
2013 Aug;43(4):198-205. 10.5051/jpis.2013.43.4.198.
Surface characteristics and bioactivity of an anodized titanium surface
- Affiliations
-
- 1Department of Periodontology, Dental Research Institute, Chonnam National University School of Dentistry, Gwangju, Korea. youngjun@chonnam.ac.kr
- KMID: 2027816
- DOI: http://doi.org/10.5051/jpis.2013.43.4.198
Abstract
- PURPOSE
The aim of this study was to evaluate the surface properties and biological response of an anodized titanium surface by cell proliferation and alkaline phosphatase activity analysis.
METHODS
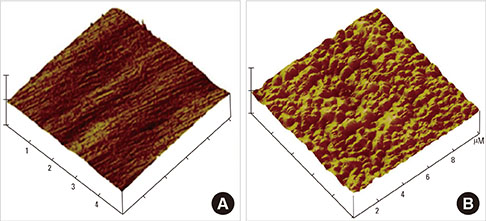
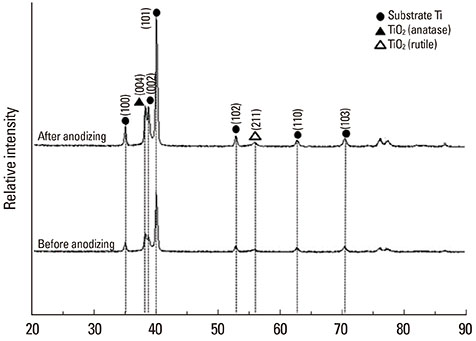
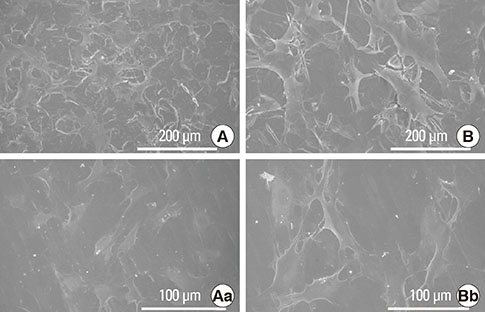
Commercial pure titanium (Ti) disks were prepared. The samples were divided into an untreated machined Ti group and anodized Ti group. The anodization of cp-Ti was formed using a constant voltage of 270 V for 60 seconds. The surface properties were evaluated using scanning electron microscopy, X-ray photoelectron spectroscopy, and an image analyzing microscope. The surface roughness was evaluated by atomic force microscopy and a profilometer. The contact angle and surface energy were analyzed. Cell adhesion, cell proliferation, and alkaline phosphatase activity were evaluated using mouse MC3T3-E1 cells.
RESULTS
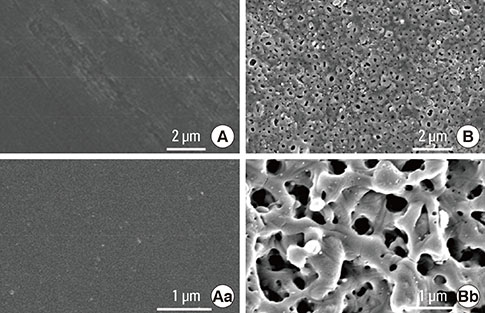
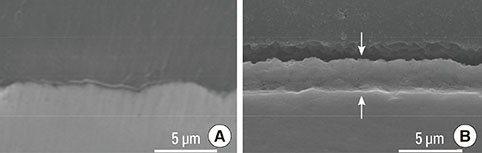
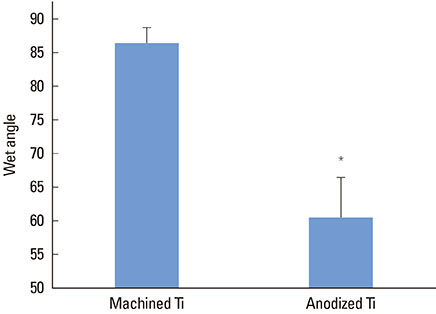
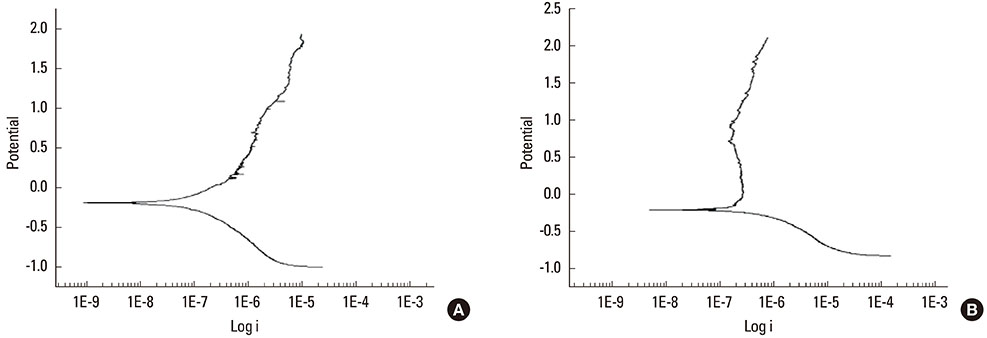
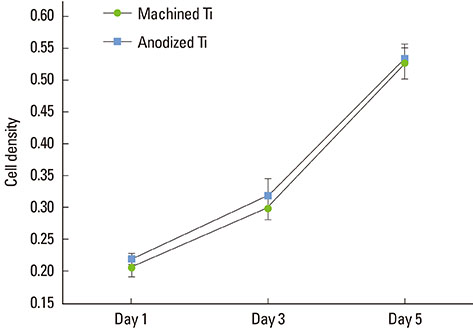
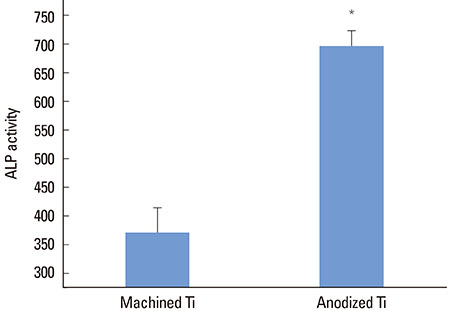
The anodized Ti group had a more porous and thicker layer on its surface. The surface roughness of the two groups measured by the profilometer showed no significant difference (P>0.001). The anodized Ti dioxide (TiO2) surface exhibited better corrosion resistance and showed a significantly lower contact angle than the machined Ti surface (P>0.001). Although there was no significant difference in the cell viability between the two groups (P>0.001), the anodized TiO2 surface showed significantly enhanced alkaline phosphatase activity (P<0.001).
CONCLUSIONS
These results suggest that the surface modification of Ti by anodic oxidation improved the osteogenic response of the osteoblast cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Uzumaki ET, Santos AR, Lambert CS. Titanium oxide (TiO2) coatings produced on titanium by oxygen plasma immersion and cell behavior on TiO2. Key Eng Mater. 2006; 18:367–370.2. Kasemo B. Biocompatibility of titanium implants: surface science aspects. J Prosthet Dent. 1983; 49:832–837.
Article3. Baier RE, Meenaghan MA, Hartman LC, Wirth JE, Flynn HE, Meyer AE, et al. Implant surface characteristics and tissue interaction. J Oral Implantol. 1988; 13:594–606.4. Ong JL, Prince CW, Raikar GN, Lucas LC. Effect of surface topography of titanium on surface chemistry and cellular response. Implant Dent. 1996; 5:83–88.
Article5. Placko HE, Mishra S, Weimer JJ, Lucas LC. Surface characterization of titanium-based implant materials. Int J Oral Maxillofac Implants. 2000; 15:355–363.6. Larsson C, Thomsen P, Lausmaa J, Rodahl M, Kasemo B, Ericson LE. Bone response to surface modified titanium implants: studies on electropolished implants with different oxide thicknesses and morphology. Biomaterials. 1994; 15:1062–1074.
Article7. Larsson C, Thomsen P, Aronsson BO, Rodahl M, Lausmaa J, Kasemo B, et al. Bone response to surface-modified titanium implants: studies on the early tissue response to machined and electropolished implants with different oxide thicknesses. Biomaterials. 1996; 17:605–616.
Article8. Lausmaa J. Mechanical, thermal, chemical and electrochemical surface treatment of titanium. In : Brunette DM, Tengvall P, Textor M, Thomsen P, editors. Titanium in medicine. New York: Springer;2011. p. 231–266.9. Sittig C, Textor M, Spencer ND, Wieland M, Vallotton PH. Surface characterization of implant materials c.p. Ti, Ti-6Al-7Nb and Ti-6Al-4V with different pretreatments. J Mater Sci Mater Med. 1999; 10:35–46.10. Bordji K, Jouzeau JY, Mainard D, Payan E, Netter P, Rie KT, et al. Cytocompatibility of Ti-6Al-4V and Ti-5Al-2.5Fe alloys according to three surface treatments, using human fibroblasts and osteoblasts. Biomaterials. 1996; 17:929–940.
Article11. Yao C, Webster TJ. Anodization: a promising nano-modification technique of titanium implants for orthopedic applications. J Nanosci Nanotechnol. 2006; 6:2682–2692.
Article12. Sato M, Webster TJ. Nanobiotechnology: implications for the future of nanotechnology in orthopedic applications. Expert Rev Med Devices. 2004; 1:105–114.
Article13. Sul YT, Johansson CB, Jeong Y, Roser K, Wennerberg A, Albrektsson T. Oxidized implants and their influence on the bone response. J Mater Sci Mater Med. 2001; 12:1025–1031.14. Sul YT, Byon ES, Jeong Y. Biomechanical measurements of calcium-incorporated oxidized implants in rabbit bone: effect of calcium surface chemistry of a novel implant. Clin Implant Dent Relat Res. 2004; 6:101–110.
Article15. Sul YT, Johansson C, Wennerberg A, Cho LR, Chang BS, Albrektsson T. Optimum surface properties of oxidized implants for reinforcement of osseointegration: surface chemistry, oxide thickness, porosity, roughness, and crystal structure. Int J Oral Maxillofac Implants. 2005; 20:349–359.16. Sul YT, Johansson C, Byon E, Albrektsson T. The bone response of oxidized bioactive and non-bioactive titanium implants. Biomaterials. 2005; 26:6720–6730.
Article17. Sunny MC, Sharma CP. Titanium-protein interaction: changes with oxide layer thickness. J Biomater Appl. 1991; 6:89–98.
Article18. Sundgren JE, I Lundstrom BP. Auger electron spectroscopic studies of the interface between human tissue and implants of titanium and stainless steel. J Colloid Interface Sci. 1986; 110:9–20.
Article19. Schreckenbach JP, Marx G, Schlottig F, Textor M, Spencer ND. Characterization of anodic spark-converted titanium surfaces for biomedical applications. J Mater Sci Mater Med. 1999; 10:453–457.20. Jeong YH, Choe HC, Brantley WA. Corrosion characteristics of anodized Ti-(10-40wt%)Hf alloys for metallic biomaterials use. J Mater Sci Mater Med. 2011; 22:41–50.
Article21. Song HJ, Kim MK, Jung GC, Vang MS, Park YJ. The effects of spark anodizing treatment of pure titanium metals and titanium alloys on corrosion characteristics. Surf Coat Technol. 2007; 201:8738–8745.
Article22. Yao C, Slamovich EB, Webster TJ. Enhanced osteoblast functions on anodized titanium with nanotube-like structures. J Biomed Mater Res A. 2008; 85:157–166.
Article23. Sul YT. The significance of the surface properties of oxidized titanium to the bone response: special emphasis on potential biochemical bonding of oxidized titanium implant. Biomaterials. 2003; 24:3893–3907.
Article24. Sul YT, Johansson CB, Jeong Y, Wennerberg A, Albrektsson T. Resonance frequency and removal torque analysis of implants with turned and anodized surface oxides. Clin Oral Implants Res. 2002; 13:252–259.
Article25. Bae IH, Yun KD, Kim HS, Jeong BC, Lim HP, Park SW, et al. Anodic oxidized nanotubular titanium implants enhance bone morphogenetic protein-2 delivery. J Biomed Mater Res B Appl Biomater. 2010; 93:484–491.
Article26. Balasundaram G, Yao C, Webster TJ. TiO2 nanotubes functionalized with regions of bone morphogenetic protein-2 increases osteoblast adhesion. J Biomed Mater Res A. 2008; 84:447–453.27. Das K, Bose S, Bandyopadhyay A. Surface modifications and cell-materials interactions with anodized Ti. Acta Biomater. 2007; 3:573–585.
Article28. Webb K, Hlady V, Tresco PA. Relative importance of surface wettability and charged functional groups on NIH 3T3 fibroblast attachment, spreading, and cytoskeletal organization. J Biomed Mater Res. 1998; 41:422–430.
Article29. Eriksson C, Nygren H, Ohlson K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials. 2004; 25:4759–4766.
Article30. Eriksson C, Nygren H. Polymorphonuclear leukocytes in coagulating whole blood recognize hydrophilic and hydrophobic titanium surfaces by different adhesion receptors and show different patterns of receptor expression. J Lab Clin Med. 2001; 137:296–302.
Article31. Oh HJ, Lee JH, Jeong Y, Kim YJ, Chi CS. Microstructural characterization of biomedical titanium oxide film fabricated by electrochemical method. Surf Coat Technol. 2005; 198:247–252.
Article32. Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, et al. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005; 74:49–58.
Article33. Lee BA, Kang CH, Vang MS, Jung YS, Piao XH, Kim OS, et al. Surface characteristics and osteoblastic cell response of alkali-and heat-treated titanium-8tantalum-3niobium alloy. J Periodontal Implant Sci. 2012; 42:248–255.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Wettability and cellular response of UV light irradiated anodized titanium surface
- Surface analyses of titanium substrate modified by anodization and nanoscale Ca-P deposition
- The effect of implant surface treated by anodizing on proliferation of the rat osteoblast
- Surface characteristics of anodic oxidized titanium according to the pore size
- Histologic evaluation and removal torque analysis of nano- and microtreated titanium implants in the dogs