Reliability of Sentinel Lymph Node Biopsy after Neoadjuvant Chemotherapy in Breast Cancer Patients
- Affiliations
-
- 1Department of Surgery, Seoul National University Hospital, Seoul, Korea. dynoh@snu.ac.kr
- 2Department of Pathology, Seoul National University Hospital, Seoul, Korea.
- 3Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1976375
- DOI: http://doi.org/10.4048/jbc.2013.16.4.378
Abstract
- PURPOSE
Sentinel lymph node biopsy (SLNB) is an accurate and effective means of axillary nodal staging in early breast cancer. However its indication after neoadjuvant chemotherapy (NAC) is under constant debate. The present study evaluates the reliability of SLNB in assessing axillary nodal status after NAC.
METHODS
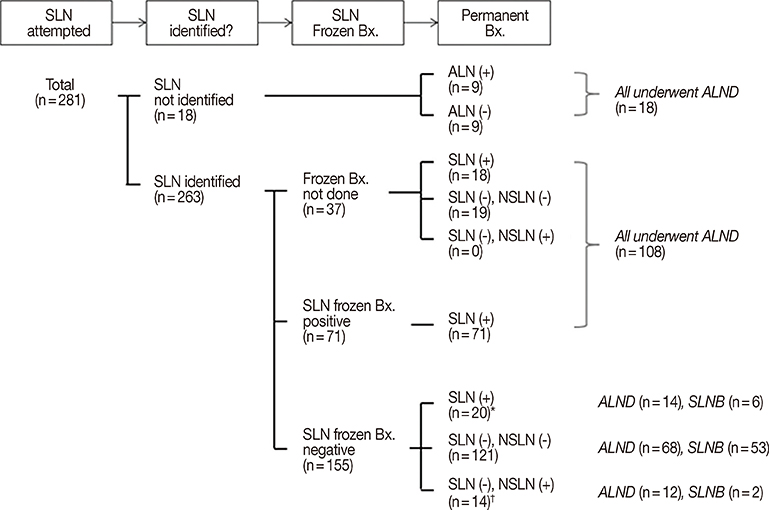
Data from 281 patients who had received NAC and subsequent SLNB were reviewed. The identification and false negative rates of SLNB were determined and the clinicopathologic factors associated with false negative results were investigated using univariate analysis.
RESULTS
The identification rate of SLNB after NAC was 93.6% and the false negative rate was 10.4%. Hormone receptor status, especially progesterone receptor positivity, was significantly associated with false negative results. The accuracy of intraoperative frozen section examination of sentinel lymph nodes was 91.2%.
CONCLUSION
The identification rate of SLNB and the accuracy of intraoperative frozen section examination after NAC are comparable to the results without NAC in patients with early breast cancer. However considering the high false negative rates, general application of SLNB after NAC should be avoided. Patients with progesterone-positive tumors and non-triple-negative breast cancers may be a select group of patients in whom SLNB can be employed safely after NAC, but further studies are necessary.
MeSH Terms
Figure
Cited by 3 articles
-
Development of a Nomogram to Predict N2 or N3 Stage in T1–2 Invasive Breast Cancer Patients with No Palpable Lymphadenopathy
Isaac Kim, Jai Min Ryu, Jai Myeong Kim, Hee Jun Choi, Se Kyung Lee, Jong Hwan Yu, Jeong Eon Lee, Seok Won Kim, Seok Jin Nam
J Breast Cancer. 2017;20(3):270-278. doi: 10.4048/jbc.2017.20.3.270.Targeted axillary biopsy and sentinel lymph node biopsy for axillary restaging after neoadjuvant chemotherapy
Gunay Gurleyik, Sibel Aydin Aksu, Fügen Aker, Kubra Kaytaz Tekyol, Eda Tanrikulu, Emin Gurleyik
Ann Surg Treat Res. 2021;100(6):305-312. doi: 10.4174/astr.2021.100.6.305.Can We Skip Intraoperative Evaluation of Sentinel Lymph Nodes? Nomogram Predicting Involvement of Three or More Axillary Lymph Nodes before Breast Cancer Surgery
Soo Kyung Ahn, Min Kyoon Kim, Jongjin Kim, Eunshin Lee, Tae-Kyung Yoo, Han-Byoel Lee, Young Joon Kang, Jisun Kim, Hyeong-Gon Moon, Jung Min Chang, Nariya Cho, Woo Kyung Moon, In Ae Park, Dong-Young Noh, Wonshik Han
Cancer Res Treat. 2017;49(4):1088-1096. doi: 10.4143/crt.2016.473.
Reference
-
1. Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer: an NSABP update. Cancer. 1983; 52:1551–1557.
Article2. Ahmed RL, Prizment A, Lazovich D, Schmitz KH, Folsom AR. Lymphedema and quality of life in breast cancer survivors: the Iowa Women's Health Study. J Clin Oncol. 2008; 26:5689–5696.
Article3. Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer. 2006; 106:4–16.
Article4. Nason KS, Anderson BO, Byrd DR, Dunnwald LK, Eary JF, Mankoff DA, et al. Increased false negative sentinel node biopsy rates after preoperative chemotherapy for invasive breast carcinoma. Cancer. 2000; 89:2187–2194.
Article5. Kuerer HM, Sahin AA, Hunt KK, Newman LA, Breslin TM, Ames FC, et al. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann Surg. 1999; 230:72–78.
Article6. Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer:National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006; 24:2019–2027.
Article7. van Deurzen CH, Vriens BE, Tjan-Heijnen VC, van der Wall E, Albregts M, van Hilligersberg R, et al. Accuracy of sentinel node biopsy after neoadjuvant chemotherapy in breast cancer patients: a systematic review. Eur J Cancer. 2009; 45:3124–3130.
Article8. Tan VK, Goh BK, Fook-Chong S, Khin LW, Wong WK, Yong WS. The feasibility and accuracy of sentinel lymph node biopsy in clinically node-negative patients after neoadjuvant chemotherapy for breast cancer: a systematic review and meta-analysis. J Surg Oncol. 2011; 104:97–103.
Article9. Fernández A, Cortés M, Benito E, Azpeitia D, Prieto L, Moreno A, et al. Gamma probe sentinel node localization and biopsy in breast cancer patients treated with a neoadjuvant chemotherapy scheme. Nucl Med Commun. 2001; 22:361–366.
Article10. Shen J, Gilcrease MZ, Babiera GV, Ross MI, Meric-Bernstam F, Feig BW, et al. Feasibility and accuracy of sentinel lymph node biopsy after preoperative chemotherapy in breast cancer patients with documented axillary metastases. Cancer. 2007; 109:1255–1263.
Article11. Lee KH, Im SA, Oh DY, Lee SH, Chie EK, Han W, et al. Prognostic significance of bcl-2 expression in stage III breast cancer patients who had received doxorubicin and cyclophosphamide followed by paclitaxel as adjuvant chemotherapy. BMC Cancer. 2007; 7:63.
Article12. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–216.13. Cho N, Moon WK, Han W, Park IA, Cho J, Noh DY. Preoperative sonographic classification of axillary lymph nodes in patients with breast cancer: node-to-node correlation with surgical histology and sentinel node biopsy results. AJR Am J Roentgenol. 2009; 193:1731–1737.
Article14. Brown AS, Hunt KK, Shen J, Huo L, Babiera GV, Ross MI, et al. Histologic changes associated with false-negative sentinel lymph nodes after preoperative chemotherapy in patients with confirmed lymph node-positive breast cancer before treatment. Cancer. 2010; 116:2878–2883.
Article15. Xing Y, Foy M, Cox DD, Kuerer HM, Hunt KK, Cormier JN. Meta-analysis of sentinel lymph node biopsy after preoperative chemotherapy in patients with breast cancer. Br J Surg. 2006; 93:539–546.
Article16. Kelly AM, Dwamena B, Cronin P, Carlos RC. Breast cancer sentinel node identification and classification after neoadjuvant chemotherapy-systematic review and meta analysis. Acad Radiol. 2009; 16:551–563.17. Classe JM, Bordes V, Campion L, Mignotte H, Dravet F, Leveque J, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: results of Ganglion Sentinelle et Chimiotherapie Neoadjuvante, a French prospective multicentric study. J Clin Oncol. 2009; 27:726–732.
Article18. Schwartz GF, Tannebaum JE, Jernigan AM, Palazzo JP. Axillary sentinel lymph node biopsy after neoadjuvant chemotherapy for carcinoma of the breast. Cancer. 2010; 116:1243–1251.
Article19. Ozmen V, Unal ES, Muslumanoglu ME, Igci A, Canbay E, Ozcinar B, et al. Axillary sentinel node biopsy after neoadjuvant chemotherapy. Eur J Surg Oncol. 2010; 36:23–29.
Article20. Reitsamer R, Menzel C, Glueck S, Rettenbacher L, Weismann C, Hutarew G. Sentinel lymph node biopsy is precise after primary systemic therapy in stage II-III breast cancer patients. Ann Surg Oncol. 2010; 17:Suppl 3. 286–290.
Article21. Kang E, Chung IY, Han SA, Kim SM, Jang M, Lyou CY, et al. Feasibility of sentinel lymph node biopsy in breast cancer patients with initial axillary lymph node metastasis after primary systemic therapy. J Breast Cancer. 2011; 14:147–152.
Article22. Pecha V, Kolarik D, Kozevnikova R, Hovorkova K, Hrabetova P, Halaska M, et al. Sentinel lymph node biopsy in breast cancer patients treated with neoadjuvant chemotherapy. Cancer. 2011; 117:4606–4616.
Article23. Canavese G, Dozin B, Vecchio C, Tomei D, Villa G, Carli F, et al. Accuracy of sentinel lymph node biopsy after neo-adjuvant chemotherapy in patients with locally advanced breast cancer and clinically positive axillary nodes. Eur J Surg Oncol. 2011; 37:688–694.
Article24. Takahashi M, Jinno H, Hayashida T, Sakata M, Asakura K, Kitagawa Y. Correlation between clinical nodal status and sentinel lymph node biopsy false negative rate after neoadjuvant chemotherapy. World J Surg. 2012; 36:2847–2852.
Article25. Alvarado R, Yi M, Le-Petross H, Gilcrease M, Mittendorf EA, Bedrosian I, et al. The role for sentinel lymph node dissection after neoadjuvant chemotherapy in patients who present with node-positive breast cancer. Ann Surg Oncol. 2012; 19:3177–3184.
Article26. Takei H, Yoshida T, Kurosumi M, Inoue K, Matsumoto H, Hayashi Y, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy predicts pathological axillary lymph node status in breast cancer patients with clinically positive axillary lymph nodes at presentation. Int J Clin Oncol. 2013; 18:547–553.
Article27. Kim SW, Han W, Park IA, Chung JK, Yeo JS, Moon WK, et al. Prospective study of 162 sentinel lymph node biopsies in breast cancer: usefulness of ultrasonography in patients selection. J Korean Breast Cancer Soc. 2003; 6:103–108.
Article28. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. The role of sentinel lymph node surgery in patients presenting with node positive breast cancer (T0-T4, N1-2) who receive neoadjuvant chemotherapy: results from the ACOSOG Z1071 trial. Cancer Res. 2012; 72:24 Suppl 3. S2–S1.29. Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012; 48:3342–3354.
Article30. Sahoo S, Lester SC. Pathology of breast carcinomas after neoadjuvant chemotherapy: an overview with recommendations on specimen processing and reporting. Arch Pathol Lab Med. 2009; 133:633–642.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sentinel Lymph Node Biopsy in Breast Cancer: A Clinical Review and Update
- Sentinel Lymph Node Biopsy in Patients with Clinically Negative Lymph Node After Neoadjuvant Chemotherapy
- The Number of Removed Lymph Nodes for an Acceptable False Negative Rate in Sentinel Lymph Node Biopsy for Breast Cancer
- Neoadjuvant Chemotherapy Decreases the Identification Rate of Sentinel Lymph Node Biopsy
- Validation and Controversy of Sentinel Node Biopsy for Breast Cancer