Cancer Res Treat.
2012 Sep;44(3):179-186.
Clinico-pathologic Parameters for Prediction of Microsatellite Instability in Colorectal Cancer
- Affiliations
-
- 1Department of Pathology, Kosin University College of Medicine, Busan, Korea.
- 2Department of Surgery, Dream Hospital, Daegu, Korea.
- 3Department of Pathology, Daegu Catholic University Medical Center, Catholic University of Daegu School of Medicine, Daegu, Korea.
- 4Department of Medical Statistics, Daegu Catholic University Medical Center, Catholic University of Daegu School of Medicine, Daegu, Korea.
- 5Department of Laboratory Medicine, Daegu Catholic University Medical Center, Catholic University of Daegu School of Medicine, Daegu, Korea. chjeon@cu.ac.kr
Abstract
- PURPOSE
Although the incidence of microsatellite instability (MSI) accounts for 10-15% of cases of colorectal cancer, its clinical application for all colorectal cancers has widened. We attempted to identify clinical and pathological parameters that may be helpful in selection of patients with MSI-high (MSI-H).
MATERIALS AND METHODS
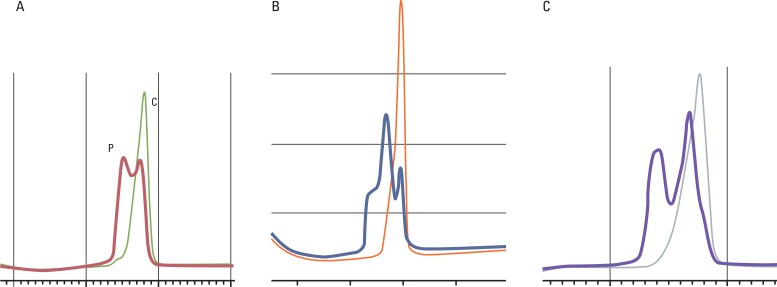
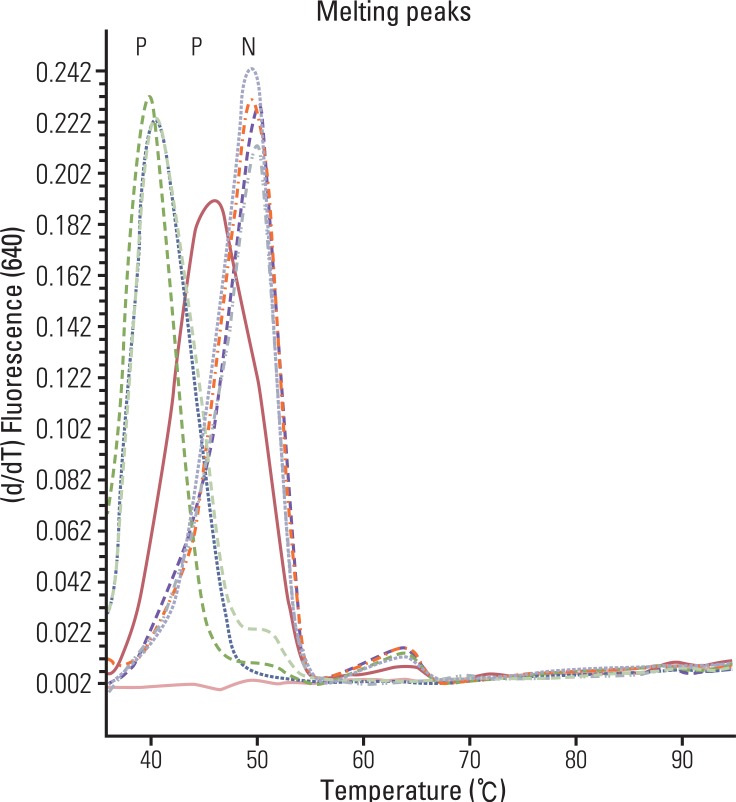
A total of 120 resected colorectal cancers were enrolled retrospectively for this MSI study. Polymerase chain reaction (PCR) and denaturing high performance liquid chromatography and/or real time PCR methods with five markers and immunohistochemistry (IHC) for MLH1 and MSH2 were performed for analysis of cancer and blood specimens. Clinico-pathologic parameters, including IHC, were investigated in order to determine their usefulness as predictive factors of MSI.
RESULTS
Among 120 cases of colorectal cancer, MSI was observed in 15 cases (12.5%), including 11 cases of MSI-H and four cases of MSI-low. Patients with MSI were younger, less than 50 years old, had a family history of cancer, Rt. sided colon cancer and/or synchronous multiple colorectal cancer, mucinous histologic type, and serum carcinoembryonic antigen group in the normal range. Results of multivariate analysis showed Bethesda guidelines, Rt. sided and/or synchronous multiple colorectal cancer, and negative expression of IHC for MLH1, which was consistently associated with MSI-H. MSI-H colorectal tumors have met at least one of these three parameters and their sensitivity and specificity were 100% and 72.5%, respectively.
CONCLUSION
Bethesda guidelines, tumor location, and negative expression of MLH1 protein are important parameters for selection of patients with colorectal cancers for MSI testing. MSI testing is recommended for patients showing any of these three parameters.
MeSH Terms
-
Carcinoembryonic Antigen
Chromatography
Chromatography, Liquid
Colonic Neoplasms
Colorectal Neoplasms
Humans
Immunohistochemistry
Incidence
Microsatellite Instability
Microsatellite Repeats
Mucins
Multivariate Analysis
Polymerase Chain Reaction
Real-Time Polymerase Chain Reaction
Reference Values
Retrospective Studies
Sensitivity and Specificity
Succinimides
Carcinoembryonic Antigen
Mucins
Succinimides
Figure
Reference
-
1. Oh SY, Lee JM, Lee HW, Kim YB, Cheong SH, Kim JM, et al. Clinicopathological significance of microsatellite instability in sporadic colorectal cancer. J Korean Surg Soc. 2006; 71:420–425.2. Sheng JQ, Zhang H, Ji M, Fu L, Mu H, Zhang MZ, et al. Genetic diagnosis strategy of hereditary non-polyposis colorectal cancer. World J Gastroenterol. 2009; 15:983–989. PMID: 19248199.
Article3. Woods MO, Younghusband HB, Parfrey PS, Gallinger S, McLaughlin J, Dicks E, et al. The genetic basis of colorectal cancer in a population-based incident cohort with a high rate of familial disease. Gut. 2010; 59:1369–1377. PMID: 20682701.
Article4. Wheeler JM, Loukola A, Aaltonen LA, Mortensen NJ, Bodmer WF. The role of hypermethylation of the hMLH1 promoter region in HNPCC versus MSI+ sporadic colorectal cancers. J Med Genet. 2000; 37:588–592. PMID: 10922385.
Article5. Watanabe T, Kanazawa T, Tada T, Kazama Y, Hata K, Nagawa H. Chemotherapy and survival in colorectal cancer patients with and without microsatellite instability: can MSI be a prognostic marker? Gastroenterology. 2004; 127:688–689. PMID: 15300610.
Article6. Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum. 1991; 34:424–425. PMID: 2022152.
Article7. Rodriguez-Bigas MA, Boland CR, Hamilton SR, Henson DE, Jass JR, Khan PM, et al. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997; 89:1758–1762. PMID: 9392616.
Article8. Alexander J, Watanabe T, Wu TT, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001; 158:527–535. PMID: 11159189.
Article9. Shia J, Ellis NA, Paty PB, Nash GM, Qin J, Offit K, et al. Value of histopathology in predicting microsatellite instability in hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer. Am J Surg Pathol. 2003; 27:1407–1417. PMID: 14576473.
Article10. Shia J, Klimstra DS, Nafa K, Offit K, Guillem JG, Markowitz AJ, et al. Value of immunohistochemical detection of DNA mismatch repair proteins in predicting germline mutation in hereditary colorectal neoplasms. Am J Surg Pathol. 2005; 29:96–104. PMID: 15613860.
Article11. Suraweera N, Duval A, Reperant M, Vaury C, Furlan D, Leroy K, et al. Evaluation of tumor microsatellite instability using five quasimonomorphic mononucleotide repeats and pentaplex PCR. Gastroenterology. 2002; 123:1804–1811. PMID: 12454837.
Article12. Dietmaier W, Hofstadter F. Detection of microsatellite instability by real time PCR and hybridization probe melting point analysis. Lab Invest. 2001; 81:1453–1456. PMID: 11598157.
Article13. Horii A, Han HJ, Shimada M, Yanagisawa A, Kato Y, Ohta H, et al. Frequent replication errors at microsatellite loci in tumors of patients with multiple primary cancers. Cancer Res. 1994; 54:3373–3375. PMID: 8012952.14. Karnes WE Jr, Shattuck-Brandt R, Burgart LJ, DuBois RN, Tester DJ, Cunningham JM, et al. Reduced COX-2 protein in colorectal cancer with defective mismatch repair. Cancer Res. 1998; 58:5473–5477. PMID: 9850081.15. Carethers JM, Chauhan DP, Fink D, Nebel S, Bresalier RS, Howell SB, et al. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology. 1999; 117:123–131. PMID: 10381918.
Article16. Sutter C, Gebert J, Bischoff P, Herfarth C, von Knebel Doeberitz M. Molecular screening of potential HNPCC patients using a multiplex microsatellite PCR system. Mol Cell Probes. 1999; 13:157–165. PMID: 10208807.
Article17. Loukola A, Eklin K, Laiho P, Salovaara R, Kristo P, Jarvinen H, et al. Microsatellite marker analysis in screening for hereditary nonpolyposis colorectal cancer (HNPCC). Cancer Res. 2001; 61:4545–4549. PMID: 11389088.18. Zhou XP, Hoang JM, Li YJ, Seruca R, Carneiro F, Sobrinho-Simoes M, et al. Determination of the replication error phenotype in human tumors without the requirement for matching normal DNA by analysis of mononucleotide repeat microsatellites. Genes Chromosomes Cancer. 1998; 21:101–107. PMID: 9491320.
Article19. Samowitz WS, Slattery ML, Potter JD, Leppert MF. BAT-26 and BAT-40 instability in colorectal adenomas and carcinomas and germline polymorphisms. Am J Pathol. 1999; 154:1637–1641. PMID: 10362787.
Article20. Salovaara R, Loukola A, Kristo P, Kaariainen H, Ahtola H, Eskelinen M, et al. Population-based molecular detection of hereditary nonpolyposis colorectal cancer. J Clin Oncol. 2000; 18:2193–2200. PMID: 10829038.
Article21. Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004; 96:261–268. PMID: 14970275.
Article22. Wullenweber HP, Sutter C, Autschbach F, Willeke F, Kienle P, Benner A, et al. Evaluation of Bethesda guidelines in relation to microsatellite instability. Dis Colon Rectum. 2001; 44:1281–1289. PMID: 11584201.23. Ward R, Meagher A, Tomlinson I, O'Connor T, Norrie M, Wu R, et al. Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut. 2001; 48:821–829. PMID: 11358903.
Article24. Engel C, Forberg J, Holinski-Feder E, Pagenstecher C, Plaschke J, Kloor M, et al. Novel strategy for optimal sequential application of clinical criteria, immunohistochemistry and microsatellite analysis in the diagnosis of hereditary nonpolyposis colorectal cancer. Int J Cancer. 2006; 118:115–122. PMID: 16003745.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Genomic Instability in Colorectal Cancer; from Bench to Bed
- Hereditary Nonpolyposis Colorectal Cancer
- Impact of Microsatellite Instability in Signet-Ring Cell and Mucinous Components in Patients With Colorectal Carcinoma
- Understanding of molecular pathogenesis and genetic markers in colorectal cancer
- Correlation MSI Status and Other Prognostic Factors in Sporadic Colorectal Cancer