Cancer Res Treat.
2008 Dec;40(4):155-163.
Radiation Therapy Combined with (or without) Cisplatin-based Chemotherapy for Patients with Nasopharyngeal Cancer: 15-years Experience of a Single Institution in Korea
- Affiliations
-
- 1Department of Radiation Oncology, The Catholic University of Korea, Seoul, Korea.
- 2Department of Diagnostic Radiology, The Catholic University of Korea, Seoul, Korea.
- 3Department of Clinical Pathology, The Catholic University of Korea, Seoul, Korea.
- 4Department of Head & Neck Surgery, The Catholic University of Korea, Seoul, Korea.
- 5Department of Medical Oncology, The Catholic University of Korea, Seoul, Korea. jinkang@catholic.ac.kr
Abstract
-
PURPOSE: This retrospective study was carried out to evaluate the efficacy and toxicity of radiation therapy (RT) with/without cisplatin-based chemotherapy in nasopharyngeal cancer (NPC).
MATERIALS AND METHODS
One hundred forty six patients with NPC received curative RT and/or cisplatin-based chemotherapy. Thirty-nine patients were treated with induction chemotherapy (IC), including cisplatin and 5-fluorouracil followed by RT. Another 63 patients were treated with concurrent chemoradiotherapy (CCRT) using cisplatin, and 22 patients were treated with IC followed by CCRT. The remaining 22 patients were treated with RT alone.
RESULTS
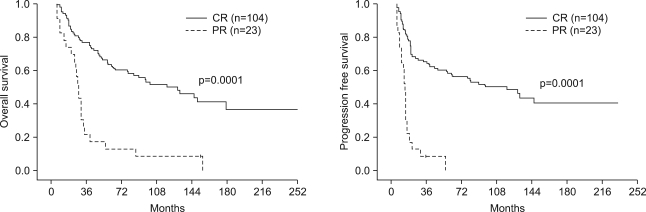
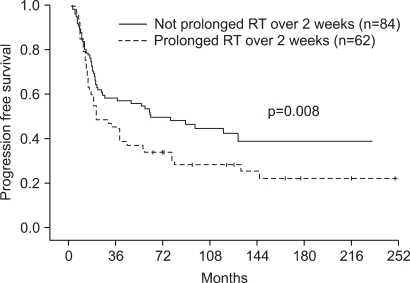
One hundred four (80.0%) patients achieved complete response (CR), and 23 (17.7%) patients achieved partial response (PR). The patterns of failure were: locoregional recurrences in 21.2% and distant metastases in 17.1%. Five-year overall survival (OS) and progression free survival (PFS) were 50.7% and 45.0%, respectively. Multivariate Cox stepwise regression analysis revealed CR to chemoradiotherapy to be a powerful prognostic factor for OS. CR to chemoradiotherapy and completion of radiation according to the time schedule were favorable prognostic factors for PFS. A comparison of each treatment group (IC --> RT vs. CCRT vs. IC --> CCRT vs. RT alone) revealed no significant differences in the OS or PFS. However, subgroup analysis showed significant differences in both OS and DFS in favor of the combined chemoradiotherapy group compared with RT alone, for stage IV and T3-4 tumors. Grade 3-4 toxicities were more common in the combined chemoradiotherapy arm, particularly in the CCRT group.
CONCLUSIONS
This study was limited in that it was a retrospective study, much time was required to collect patients, and there were imbalances in the number of patients in each treatment group. Combined chemoradiotherapy remarkably prolonged the OS and PFS in subgroup patients with stage IV or T3-4 NPC.
MeSH Terms
Figure
Reference
-
1. Altun M, Fandi A, Dupuis O, Cvitkovic E, Krajina Z, Eschwege F. Undifferentiated nasopharyngeal cancer (UCNT): Current diagnostic and therapeutic aspects. Int J Radiat Oncol Biol Phys. 1995; 32:859–877. PMID: 7790274.
Article2. Ho JH. An epidemiologic and clinical study of nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1978; 4:182–189. PMID: 640889.
Article3. Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wanh WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression free survival. J Clin Oncol. 2003; 21:631–637. PMID: 12586799.4. Teo P, Yu P, Lee WY, Leung SF, Kwan WH, Yu KH, et al. Significant prognosticators after primary radiotherapy in 903 nondisseminated nasopharyngeal carcinoma evaluated by computer tomography. Int J Radiat Oncol Biol Phys. 1996; 36:291–304. PMID: 8892451.
Article5. Chan AT, Teo PM, Ngan RK, Leung TW, Lau WH, Zee B, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of phase III randomized trial. J Clin Oncol. 2002; 20:2038–2044. PMID: 11956263.6. Ali H, Al-Sarraf M. Chemotherapy in advanced nasopharyngeal cancer. Oncology. 2000; 14:1223–1230. PMID: 10989829.7. Chan AT, Teo PM, Leung TW, Leung SF, Lee WY, Yeo W, et al. A prospective randomized study of chemotherapy adjunctive to definitive radiotherapy in advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1995; 33:569–577. PMID: 7558945.
Article8. Rossi A, Molinari R, Boracchi P, Del Vecchio M, Marubini E, Nava M, et al. Adjuvant chemotherapy with vincristine, cyclophosphamide, and doxorubicin after radiotherapy in local-regional nasopharyngeal cancer: Results of a 4-year multicenter randomized study. J Clin Oncol. 1988; 6:1401–1410. PMID: 3047335.
Article9. International Nasopharynx Cancer Study Group. Preliminary results of a randomized trial comparing neoadjuvant chemotherapy (cisplatin, epirubicin, bleomycin) plus radiotherapy vs. radiotherapy alone in stage IV (≥N2, M0) undifferentiated nasopharyngeal carcinoma: A positive effect on progression-free survival. Int J Radiat Oncol Biol Phys. 1996; 35:463–469. PMID: 8655368.10. Chua DT, Sham JS, Choy D, Lorvidhaya V, Sumitsawan Y, Thongprasert S, et al. Preliminary report of the Asian-Oceanian Clinical Oncology Association randomized trial comparing cisplatin and epirubicin followed by radiotherapy versus radiotherapy alone in the treatment of patients with locoregionally advanced nasopharyngeal carcinoma. Cancer. 1998; 83:2270–2283. PMID: 9840526.
Article11. Huncharek M, Kupelnick B. Combined chemoradiation versus radiation therapy alone in locally advanced nasopharyngeal carcinoma: results of a meta-analysis of 1,528 patients from six randomized trials. Am J Clin Oncol. 2002; 25:219–223. PMID: 12040275.12. Baujat B, Audry H, Bourhis J, Chan AT, Onat H, Chua DT, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: An individual patient data meta-analysis of eight randomized trials and 1,753 patients. Int J Radiat Oncol Biol Phys. 2006; 64:47–56. PMID: 16377415.
Article13. Boussen H, Cvitkovic E, Wendling JL, Azli N, Bachouchi M, Mahjoubi R, et al. Chemotherapy of metastatic and/or recurrent undifferentiated nasopharyngeal carcinoma with cisplatin, bleomycin, and fluorouracil. J Clin Oncol. 1991; 9:1675–1681. PMID: 1714951.
Article14. Al-Sarraf M, LeBlanc M, Giri PGS, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup Study 0099. J Clin Oncol. 1998; 16:1310–1317. PMID: 9552031.
Article15. Ma J, Mai HQ, Hong MH, Min HQ, Mao ZD, Cui NJ, et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol. 2001; 19:1350–1357. PMID: 11230478.
Article16. Tan EH, Chau ET, Wee J, Tan T, Fong KW, Ang PT, et al. Concurrent chemoradiotherapy followed by adjuvant chemotherapy in Asian patients with nasopharyngeal carcinoma: toxicities and preliminary results. Int J Radiat Oncol Biol Phys. 1999; 45:597–601. PMID: 10524411.
Article17. Lee AW, Lau WH, Tung SY, Chua DT, Chappell R, Xu L, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally advanced nasopharyngeal carcinoma: NPC-9901 trial by the Hong Kong nasopharyngeal carcinoma study group. J Clin Oncol. 2005; 23:6966–6975. PMID: 16192584.18. Wee J, Tan EH, Tai BC, Wong HB, Leong SS, Tan T, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with AJCC/IUAC stage III and IV nasopharyngeal cancer of the endemic varieties. J Clin Oncol. 2005; 23:6730–6738. PMID: 16170180.19. Bachaud JM, Cohen-Jonathan E, Alzieu C, David JM, Serrano E, Daly-Schveitzer N. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma: Final report of a randomized trial. Int J Radiat Oncol Biol Phys. 1996; 36:999–1004. PMID: 8985019.
Article20. Kim TH, Ko YH, Lee MA, Kim BS, Chung SR, Yoo IR, et al. Treatment outcome of cisplatin-based concurrent chemoradiotherapy in the patients with locally advanced nasopharyngeal cancer. Cancer Res Treat. 2008; 40:62–70.
Article21. Hsu MM, Tu SM. Nasopharyngeal carcinoma in Taiwan: Clinical manifestations and results of therapy. Cancer. 1983; 52:362–368. PMID: 6190547.
Article22. Lee AW, Poon YF, Foo W, Law SC, Cheung FK, Chan DK, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976-1985: Overall survival and patterns of failure. Int J Radiat Oncol Biol Phys. 1992; 23:261–270. PMID: 1587745.
Article23. Vikram B, Mishra UB, Strong EW, Manolatos S. Patterns of failure in carcinoma of the nasopharynx: Failure at the primary site. Int J Radiat Oncol Biol Phys. 1985; 11:1455–1459. PMID: 3926733.24. Kwong D, Sham J, Choy D. The effect of loco-regional control on distant metastatic dissemination in the carcinoma of the nasopharynx: An analysis of 1301 patients. Int J Radiat Oncol Biol Phys. 1994; 30:1029–1036. PMID: 7961008.25. Langendijk JA, Buter J, Leemans CR. A meta-analysis on the value of chemotherapy in nasopharyngeal carcinoma: An update. Otorinolaryngologie a Foiatrie, [abstract]. 2006. 55:International Federation of Head and Neck Oncologic Society;p. 017.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neoadjuvant Chemotherapy and Radiation Therapy inAdvanced Stage Nasopharyngeal Carcinoma
- Sequential Chemotherapy and Radiation Therapy for Advanced Nasopharyngeal Carcinoma
- Treatment of Small Cell Lung Cancer
- Prognostic Significance of Expression of Glutathione S-Transferase-pi and Multidrug Resistance-Associated Protein in Nasopharyngeal Carcinoma
- Less is more: role of additional chemotherapy to concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal cancer management