Allergy Asthma Immunol Res.
2012 Jul;4(4):231-239. 10.4168/aair.2012.4.4.231.
Expression and Roles of MMP-2, MMP-9, MMP-13, TIMP-1, and TIMP-2 in Allergic Nasal Mucosa
- Affiliations
-
- 1Department of Otorhinolaryngology, Nippon Medical School, Tokyo, Japan. pawankar.ruby@gmail.com
- 2Department of Pediatrics, Nippon Medical School, Tokyo, Japan.
- KMID: 1970712
- DOI: http://doi.org/10.4168/aair.2012.4.4.231
Abstract
- PURPOSE
Allergic rhinitis (AR) and asthma share many characteristics, but structural changes are observed far less often in AR. Matrix metalloproteinases (MMPs) constitute a family of Zn-dependent endopeptidases that can decompose the extracellular matrix and basement membrane, and regulate cell infiltration. We analyzed the expression of MMPs and their inhibitors, tissue inhibitors of metalloproteinases (TIMPs), in allergic nasal mucosa after nasal allergen challenge (NAC) and determined their relationship to inflammatory cells.
METHODS
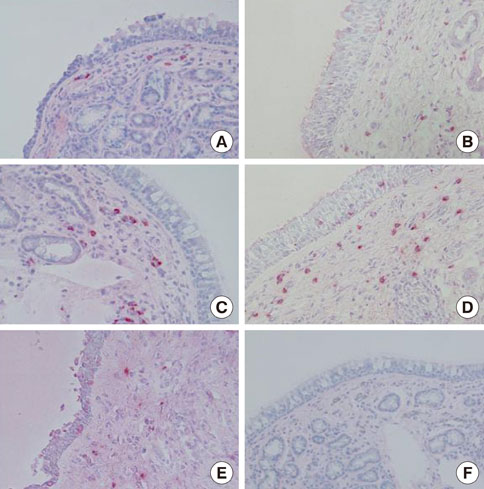
Nasal mucosa specimens were obtained at surgery performed for hypertrophied turbinates. We performed NAC with house dust mite (HDM) allergen disks and control disks, and took biopsies at 30 minutes, 6 hours, and 12 hours after NAC. Cells expressing MMP-2, MMP-9, MMP-13, TIMP-1, and TIMP-2, as well as eosinophils and mast cells, were analyzed immunohistochemically. The MMPs and TIMPs in allergic nasal mucosa were quantified using enzyme-linked immunosorbent assays.
RESULTS
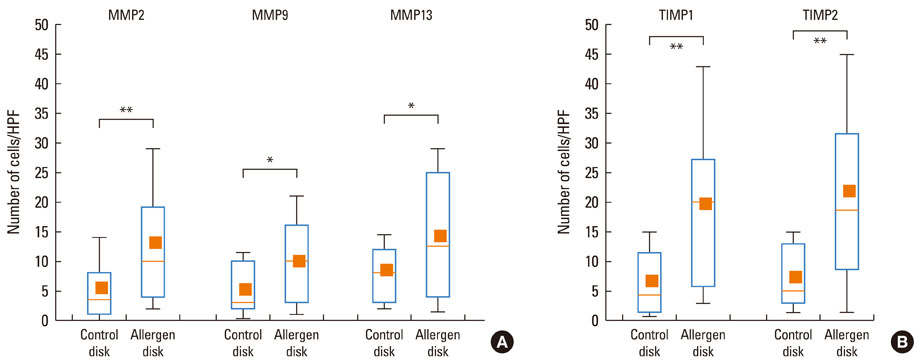
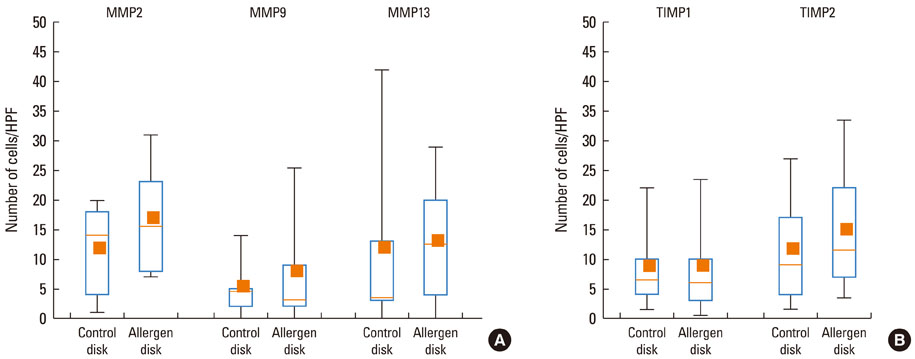
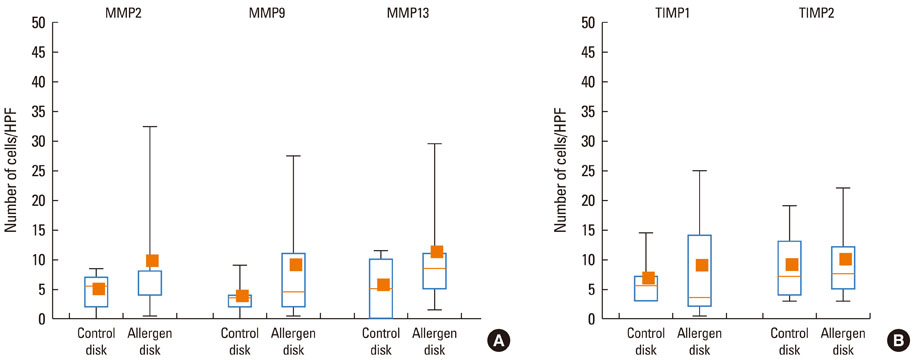
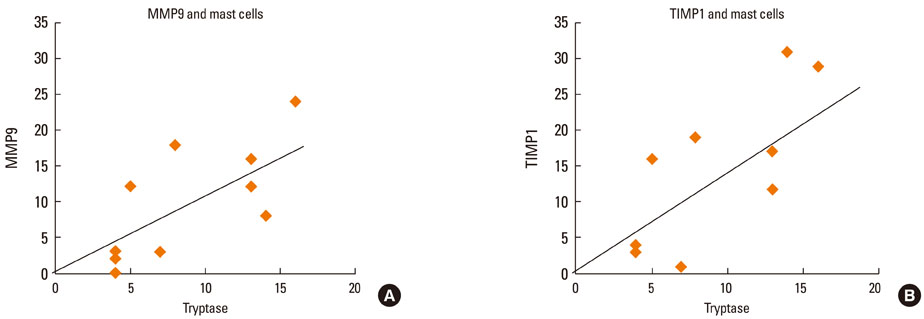
At 30 minutes post-NAC, HDM-exposed nasal mucosa exhibited significantly more MMP-2+, MMP-9+, MMP-13+, TIMP-1+, and TIMP-2+ cells compared with control mucosa, and the numbers of MMP-9+ and TIMP-1+ cells correlated strongly with the number of mast cells. At 6 hours post-NAC, the numbers of MMP+ and TIMP+ cells did not differ significantly between HDM-exposed mucosa and control mucosa, but the ratios of MMP+ cells to TIMP+ cells were higher in HDM-exposed mucosa. At 12 hours post-NAC, the number of MMP-13+ cells tended to be higher in HDM-exposed mucosa and was strongly correlated with the number of eosinophils. Quantitatively, the levels of MMP-2 and MMP-13 were significantly higher than the MMP-9 level, and the TIMP-2 level was significantly higher than the TIMP-1 level in allergic nasal mucosa.
CONCLUSIONS
We demonstrated increased expression of MMP-2, MMP-9, and MMP-13 in allergic nasal mucosa, high MMPs-to-TIMP-1 ratios, and a strong correlation between MMP-9 and mast cells and between MMP-13 and eosinophils. The imbalance between MMPs and TIMPs may contribute to the migration of inflammatory cells such as eosinophils and mast cells to the nasal mucosa of AR patients, suggesting a possible active role of MMPs in AR.
Keyword
MeSH Terms
-
Asthma
Basement Membrane
Biopsy
Endopeptidases
Eosinophils
Extracellular Matrix
Humans
Mast Cells
Matrix Metalloproteinases
Metalloproteases
Mucous Membrane
Nasal Mucosa
Pyroglyphidae
Rhinitis
Rhinitis, Allergic, Perennial
Tissue Inhibitor of Metalloproteinase-1
Tissue Inhibitor of Metalloproteinase-2
Turbinates
Endopeptidases
Matrix Metalloproteinases
Metalloproteases
Tissue Inhibitor of Metalloproteinase-1
Tissue Inhibitor of Metalloproteinase-2
Figure
Reference
-
1. Howarth PH. ABC of allergies. Pathogenic mechanisms: a rational basis for treatment. BMJ. 1998. 316:758–761.2. Howarth PH, Salagean M, Dokic D. Allergic rhinitis: not purely a histamine-related disease. Allergy. 2000. 55:Suppl 64. 7–16.3. Pawankar R. Allergic rhinitis and asthma: are they manifestations of one syndrome? Clin Exp Allergy. 2006. 36:1–4.4. Djukanović R, Wilson SJ, Howarth PH. Pathology of rhinitis and bronchial asthma. Clin Exp Allergy. 1996. 26:Suppl 3. 44–51.5. Mygind N, Bisgaard H. Mygind N, Pipkorn U, Dahl R, editors. Applied anatomy of the airways. Rhinitis and asthma: similarities and differences. 1990. Copenhagen: Munksgaard;21–37.6. Busse WW, Holgate ST, editors. Asthma and rhinitis. 1995. Boston: Blackwell Scientific Publications;12–624.7. Lee KS, Jin SM, Kim HJ, Lee YC. Matrix metalloproteinase inhibitor regulates inflammatory cell migration by reducing ICAM-1 and VCAM-1 expression in a murine model of toluene diisocyanate-induced asthma. J Allergy Clin Immunol. 2003. 111:1278–1284.8. Hoshino M, Nakamura Y, Sim J, Shimojo J, Isogai S. Bronchial subepithelial fibrosis and expression of matrix metalloproteinase-9 in asthmatic airway inflammation. J Allergy Clin Immunol. 1998. 102:783–788.9. Murphy G, Docherty AJ. The matrix metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol. 1992. 7:120–125.10. Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997. 378:151–160.11. Howarth PH. The cellular basis for allergic rhinitis. Allergy. 1995. 50:6–10.12. Salib RJ, Howarth PH. Remodelling of the upper airways in allergic rhinitis: is it a feature of the disease? Clin Exp Allergy. 2003. 33:1629–1633.13. Shimizu T, Kanai K, Asano K, Hisamitsu T, Suzaki H. Suppression of matrix metalloproteinase production in nasal fibroblasts by tranilast, an antiallergic agent, in vitro. Mediators Inflamm. 2005. 2005:150–159.14. Hoshino M, Takahashi M, Takai Y, Sim J. Inhaled corticosteroids decrease subepithelial collagen deposition by modulation of the balance between matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 expression in asthma. J Allergy Clin Immunol. 1999. 104:356–363.15. Ohno I, Ohtani H, Nitta Y, Suzuki J, Hoshi H, Honma M, Isoyama S, Tanno Y, Tamura G, Yamauchi K, Nagura H, Shirato K. Eosinophils as a source of matrix metalloproteinase-9 in asthmatic airway inflammation. Am J Respir Cell Mol Biol. 1997. 16:212–219.16. Kanbe N, Tanaka A, Kanbe M, Itakura A, Kurosawa M, Matsuda H. Human mast cells produce matrix metalloproteinase 9. Eur J Immunol. 1999. 29:2645–2649.17. Fang KC, Wolters PJ, Steinhoff M, Bidgol A, Blount JL, Caughey GH. Mast cell expression of gelatinases A and B is regulated by kit ligand and TGF-beta. J Immunol. 1999. 162:5528–5535.18. Vignola AM, Chiappara G, Chanez P, Merendino AM, Pace E, Spatafora M, Bousquet J, Bonsignore G. Growth factors in asthma. Monaldi Arch Chest Dis. 1997. 52:159–169.19. Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989. 1:520–524.20. Chakir J, Laviolette M, Boutet M, Laliberté R, Dubé J, Boulet LP. Lower airways remodeling in nonasthmatic subjects with allergic rhinitis. Lab Invest. 1996. 75:735–744.21. Bousquet J, Jacot W, Vignola AM, Bachert C, Van Cauwenberge P. Allergic rhinitis: a disease remodeling the upper airways? J Allergy Clin Immunol. 2004. 113:43–49.22. Pawankar R, Watanabe S, Nonaka M, Ozu C, Aida M, Yagi T. Differential expression of matrix metalloproteinase 2 and 9 in the allergic nasal mucosa and nasal polyps. J Allergy Clin Immunol. 2004. 113:S332.23. Calderón MA, Lozewicz S, Prior A, Jordan S, Trigg CJ, Davies RJ. Lymphocyte infiltration and thickness of the nasal mucous membrane in perennial and seasonal allergic rhinitis. J Allergy Clin Immunol. 1994. 93:635–643.24. Pawankar R. Inflammatory mechanisms in allergic rhinitis. Curr Opin Allergy Clin Immunol. 2007. 7:1–4.25. Shaida A, Kenyon G, Devalia J, Davies RJ, MacDonald TT, Pender SL. Matrix metalloproteinases and their inhibitors in the nasal mucosa of patients with perennial allergic rhinitis. J Allergy Clin Immunol. 2001. 108:791–796.26. Herouy Y, Mellios P, Bandemir E, Dichmann S, Nockowski P, Schöpf E, Norgauer J. Inflammation in stasis dermatitis upregulates MMP-1, MMP-2 and MMP-13 expression. J Dermatol Sci. 2001. 25:198–205.27. Dahlen B, Shute J, Howarth P. Immunohistochemical localisation of the matrix metalloproteinases MMP-3 and MMP-9 within the airways in asthma. Thorax. 1999. 54:590–596.28. Shin HW, Han DH, Lim YS, Kim HJ, Kim DY, Lee CH, Min YG, Rhee CS. Nonasthmatic nasal polyposis patients with allergy exhibit greater epithelial MMP positivity. Otolaryngol Head Neck Surg. 2009. 141:442–447.29. Moon IJ, Kim DY, Rhee CS, Lee CH, Min YG. Role of angiogenic factors in airway remodeling in an allergic rhinitis murine model. Allergy Asthma Immunol Res. 2012. 4:37–45.30. Erlewyn-Lajeunesse MD, Hunt LP, Pohunek P, Dobson SJ, Kochhar P, Warner JA, Warner JO. Bronchoalveolar lavage MMP-9 and TIMP-1 in preschool wheezers and their relationship to persistent wheeze. Pediatr Res. 2008. 64:194–199.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of MMP-9 and TIMP-1 in the Nasal Mucosa of Allergic Rhinitis
- Expression of Matrix Metalloproteinase-2 (MMP-2) and Tissue Inhibitor of Metalloproteinase-2 (TIMP-2) in Pancreatic Ductal Adenocarcinoma
- Immunodetections of the Metalloproteinase (MMP-2 and MMP-9) and Tissue Inhibitor of Metalloproteinases (TIMP-2) in Prostatic Adenocarcinomas
- MMP-2 and MMP-9 Expressions in Breast Carcinomas and Relationship with Major Prognostic Factors
- Expression of MMP-2, MT1-MMP, and TIMP-2 mRNA in Breast Carcinomas