Clin Endosc.
2015 Jul;48(4):336-339. 10.5946/ce.2015.48.4.336.
A Patient with Duodenal Mucinous Adenocarcinoma Presenting as a Laterally Spreading Tumor
- Affiliations
-
- 1Department of Gastroenterology, Kyung Hee University Hospital at Gangdong, Kyung Hee University School of Medicine, Seoul, Korea. topcolon@nate.com
- KMID: 1964279
- DOI: http://doi.org/10.5946/ce.2015.48.4.336
Abstract
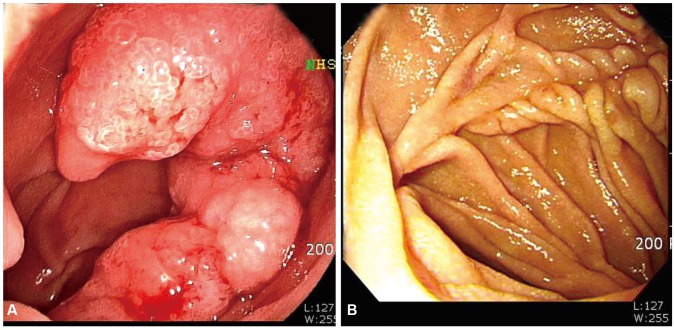
- Primary duodenal carcinoma is rare. Duodenal mucinous adenocarcinoma (DMA) is even rarer, and its associated manifestations and typical endoscopic or imaging findings are not well characterized. Herein, we report a case of primary DMA in an asymptomatic 58-year-old man who visited our hospital for a regular health screening. Upper endoscopy revealed an approximately 4-cm lesion in the second portion of the duodenum, but the mass was not visualized on computed tomography. Biopsies revealed a tubular adenoma that was subsequently resected. Frozen biopsies demonstrated DMA with a background of low-grade tubular adenoma for which we performed Roux-en-Y duodenojejunostomy and jejunojejunostomy. To our knowledge, this is the first report of a patient with DMA in Korea.
Keyword
MeSH Terms
Figure
Reference
-
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014; 64:9–29. PMID: 24399786.
Article2. Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009; 249:63–71. PMID: 19106677.3. Yagyu T, Aihara T, Murayama M, et al. Mucinous carcinoma of the duodenum associated with hereditary nonpolyposis colorectal cancer: report of a case. Surg Today. 2006; 36:1129–1132. PMID: 17123147.
Article4. Inagaki M, Obara M, Suzuki S, et al. Mucinous carcinoma of Vater's ampulla with a unique extension along the main pancreatic duct. J Hepatobiliary Pancreat Surg. 2007; 14:518–521. PMID: 17909724.
Article5. Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004; 101:518–526. PMID: 15274064.6. Aparicio T, Zaanan A, Svrcek M, et al. Small bowel adenocarcinoma: epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis. 2014; 46:97–104. PMID: 23796552.
Article7. Kummar S, Ciesielski TE, Fogarasi MC. Management of small bowel adenocarcinoma. Oncology (Williston Park). 2002; 16:1364–1369. PMID: 12435206.8. Faivre J, Trama A, De Angelis R, et al. Incidence, prevalence and survival of patients with rare epithelial digestive cancers diagnosed in Europe in 1995-2002. Eur J Cancer. 2012; 48:1417–1424. PMID: 22169462.
Article9. Chow JS, Chen CC, Ahsan H, Neugut AI. A population-based study of the incidence of malignant small bowel tumours: SEER, 1973-1990. Int J Epidemiol. 1996; 25:722–728. PMID: 8921448.
Article10. Michelassi F, Testa G, Pomidor WJ, Lashner BA, Block GE. Adenocarcinoma complicating Crohn's disease. Dis Colon Rectum. 1993; 36:654–661. PMID: 8348849.
Article11. Halfdanarson TR, McWilliams RR, Donohue JH, Quevedo JF. A single-institution experience with 491 cases of small bowel adenocarcinoma. Am J Surg. 2010; 199:797–803. PMID: 20609724.
Article12. Abrahams NA, Halverson A, Fazio VW, Rybicki LA, Goldblum JR. Adenocarcinoma of the small bowel: a study of 37 cases with emphasis on histologic prognostic factors. Dis Colon Rectum. 2002; 45:1496–1502. PMID: 12432298.13. Jass JR, Sobin LH. Histologic Typing of Intestinal Tumours. 2nd ed. Berlin: Springer Verlag;1989.14. Yasuda K, Adachi Y, Shiraishi N, Yamaguchi K, Shiromizu A, Kitano S. Pathology and prognosis of mucinous gastric carcinoma. J Surg Oncol. 2001; 76:272–277. PMID: 11320519.
Article15. Verhulst J, Ferdinande L, Demetter P, Ceelen W. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol. 2012; 65:381–388. PMID: 22259177.
Article16. Hirota E, Ochiai A, Oda Y, et al. Significance of histological type of gastric carcinoma as a prognostic factor. Stomach Intest. 1991; 26:1149–1158.17. Adachi Y, Mori M, Kido A, Shimono R, Maehara Y, Sugimachi K. A clinicopathologic study of mucinous gastric carcinoma. Cancer. 1992; 69:866–871. PMID: 1310432.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Duodenal Mucinous Carcinoma: A Case Report
- A Case of Mucinous Gastric Adenocarcinoma as Submucosal Tumor
- Ovarian Mucinous Adenocarcinoma Associated with Mucinous Adenocarcinoma of the Uterine Cervix
- A Case of Primary Mucinous Adenocarcinoma of the Lateral Lower Eyelid
- Two cases of mucinous adenocarcinoma of the stomach mistaken as submucosal tumor