Prog Med Phys.
2014 Sep;25(3):157-166. 10.14316/pmp.2014.25.3.157.
Effect of ATP on Calcium Channel Modulation in Rat Adrenal Chromaffin Cells
- Affiliations
-
- 1Department of Biomedical Engineering, Chungbuk National University School of Medicine, Cheongju, Korea.
- 2Department of Physiology, Chungbuk National University School of Medicine, Cheongju, Korea. ysgoo@chungbuk.ac.kr
- KMID: 1910548
- DOI: http://doi.org/10.14316/pmp.2014.25.3.157
Abstract
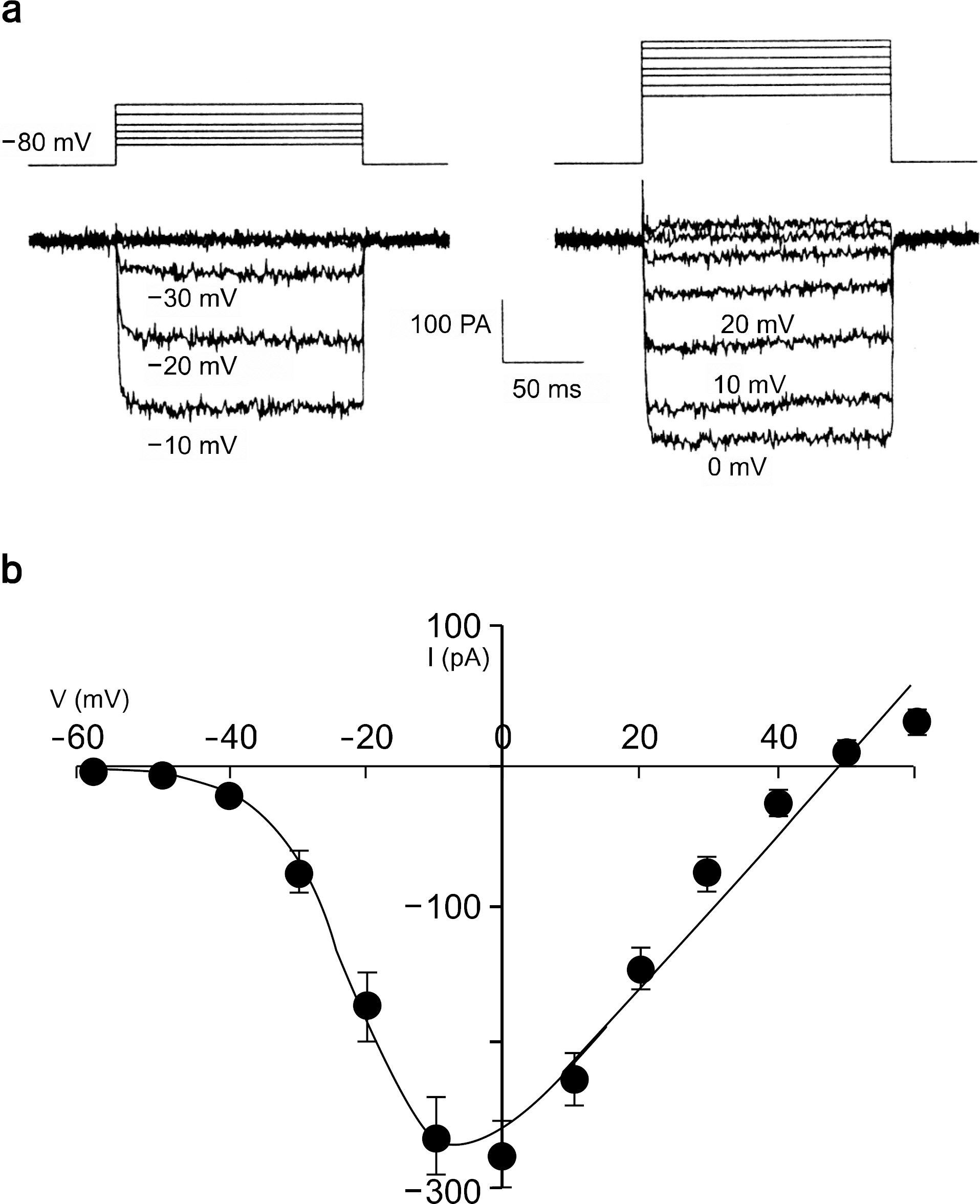
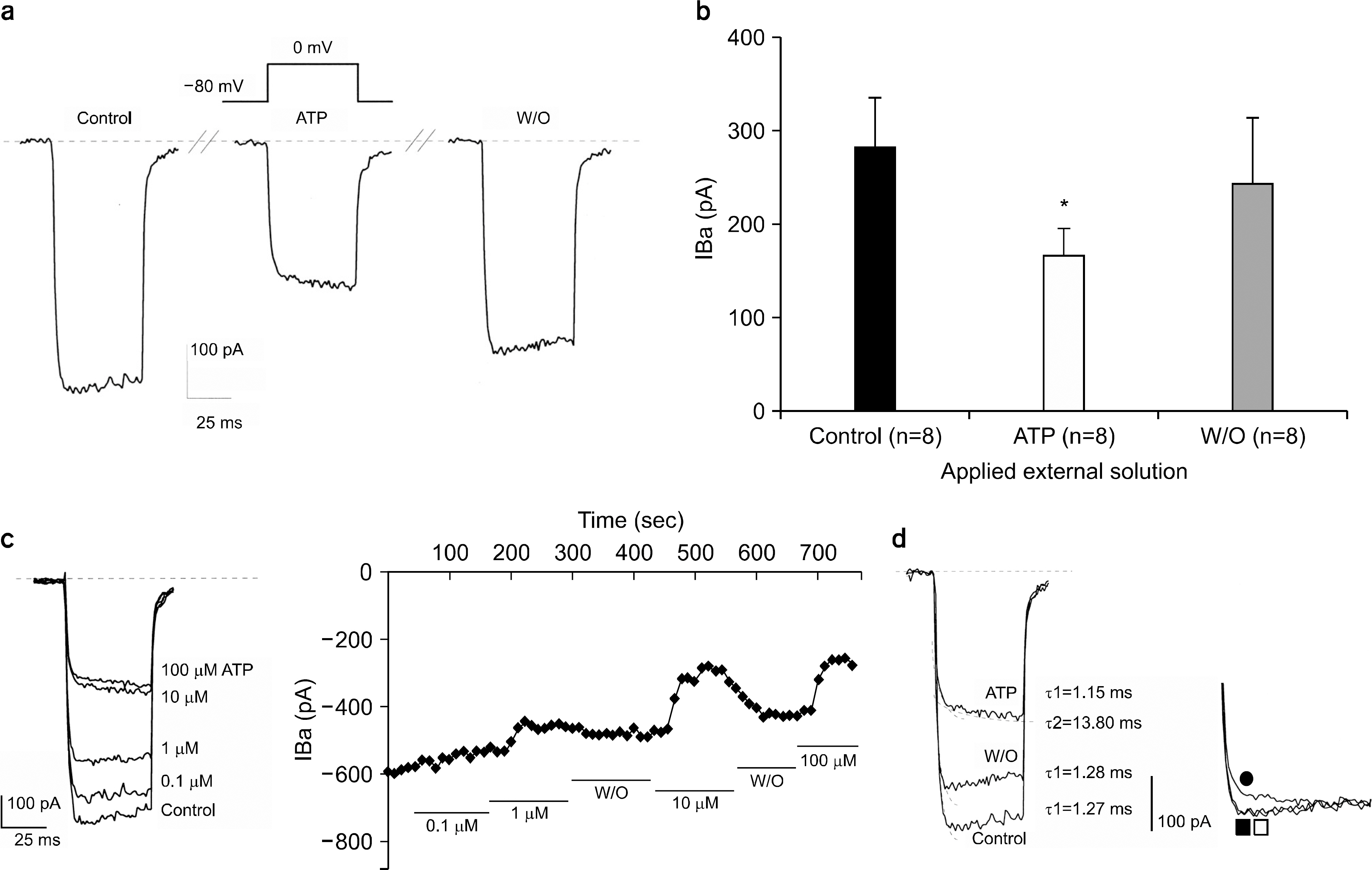
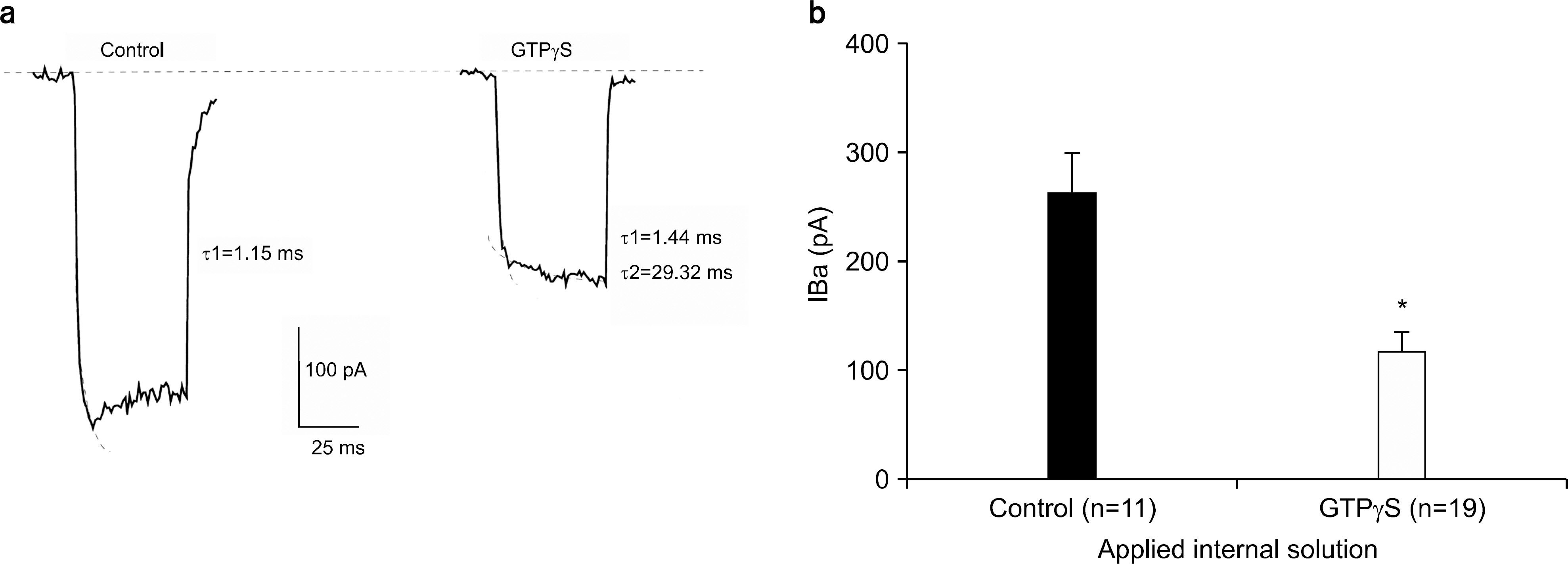
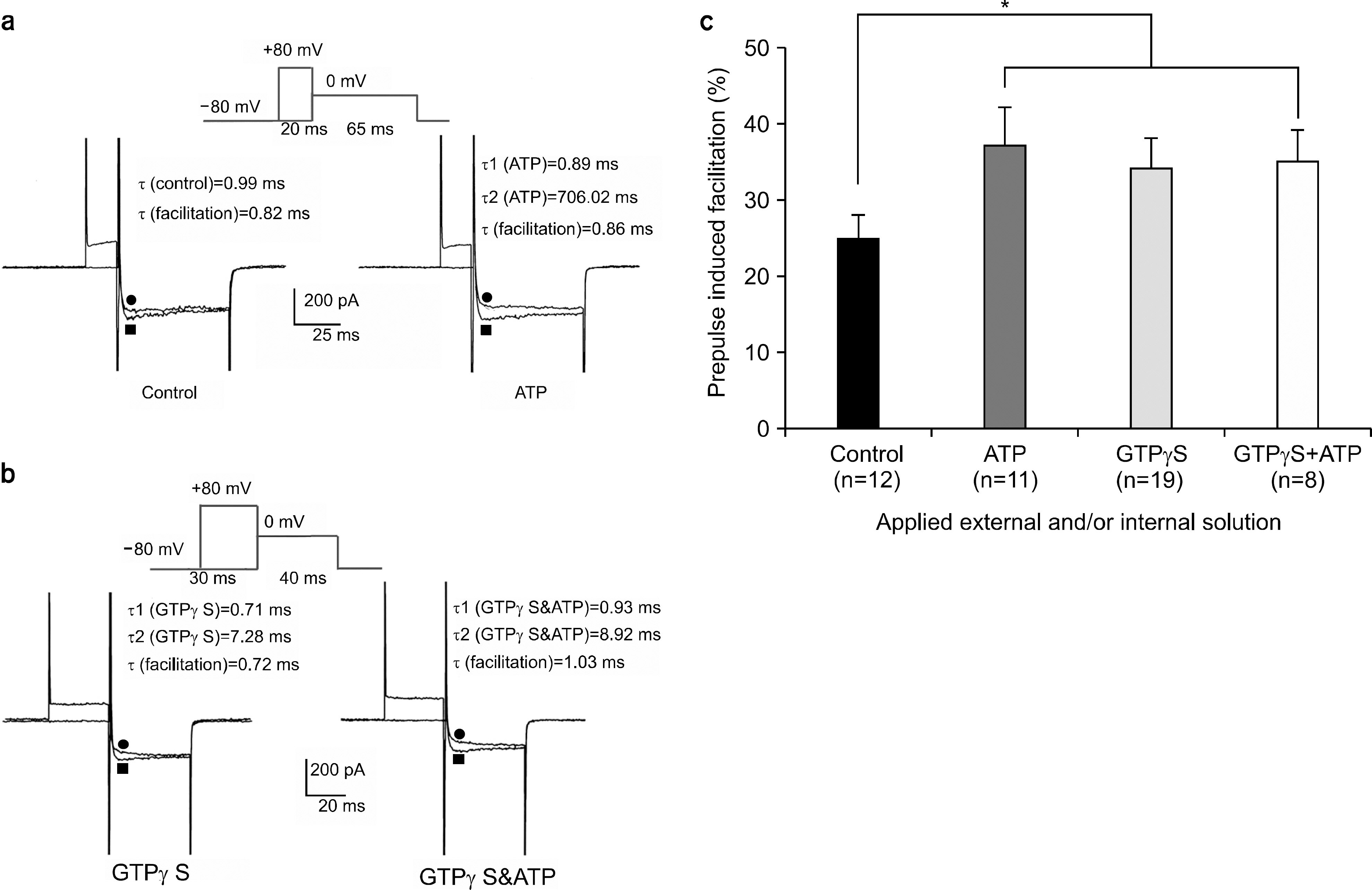
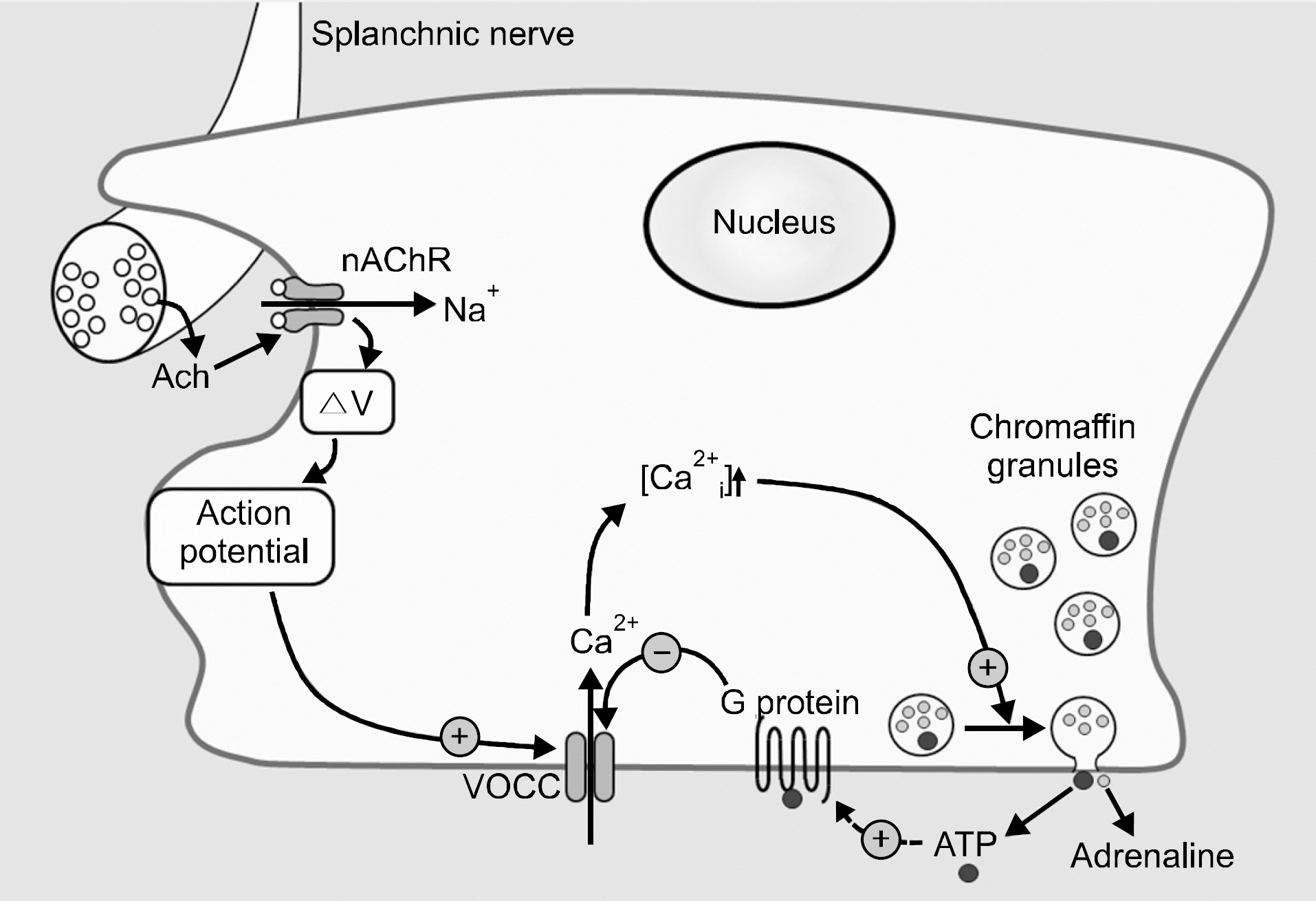
- ATP in quantity co-stored with neurotransmitters in the secretory vesicles of neurons, by being co-released with the neurotransmitters, takes an important role to modulate the stimulus-secretion response of neurotransmitters. Here, in this study, the modulatory effect of ATP was studied in Ca2+ channels of cultured rat adrenal chromaffin cells to investigate the physiological role of ATP in neurons. The Ca2+ channel current was recorded in a whole-cell patch clamp configuration, which was modulated by ATP. In 10 mM Ba2+ bath solution, ATP treatment (0.1 mM) decreased the Ba2+ current by an average of 36+/-6% (n=8), showing a dose-dependency within the range of 10(-4)~10(-1) mM. The current was recovered by ATP washout, demonstrating its reversible pattern. This current blockade effect of ATP was disinhibited by a large prepulse up to +80 mV, since the Ba2+ current increment was larger when treated with ATP (37+/-5%, n=11) compared to the control (25+/-3%, n=12, without ATP). The Ba2+ current was recorded with GTPgammaS, the non-hydrolyzable GTP analogue, to determine if the blocking effect of ATP was mediated by G-protein. The Ba2+ current decreased down to 45% of control with GTPgammaS. With a large prepulse (+80 mV), the current increment was 34+/-4% (n=19), which 25+/-3% (n=12) under control condition (without GTPgammaS). The Ba2+ current waveform was well fitted to a single-exponential curve for the control, while a double-exponential curve best fitted the current signal with ATP or GTPgammaS. In other words, a slow activation component appeared with ATP or GTPgammaS, which suggested that both ATP and GTPgammaS caused slower activation of Ca2+ channels via the same mechanism. The results suggest that ATP may block the Ca2+ channels by G-protein and this Ca2+ channel blocking effect of ATP is important in autocrine (or paracrine) inhibition of adrenaline secretion in chromaffin cell.
MeSH Terms
-
Adenosine Triphosphate*
Animals
Baths
Calcium Channels*
Chromaffin Cells*
Epinephrine
GTP-Binding Proteins
Guanosine 5'-O-(3-Thiotriphosphate)
Guanosine Triphosphate
Neurons
Neurotransmitter Agents
Rats*
Secretory Vesicles
Adenosine Triphosphate
Calcium Channels
Epinephrine
GTP-Binding Proteins
Guanosine 5'-O-(3-Thiotriphosphate)
Guanosine Triphosphate
Neurotransmitter Agents
Figure
Reference
-
References
1. Augustine GJ, Neher E. Calcium requirments for secretion in bovine chromaffin cells. J Physiol. 450:247–271. 1992.2. Boarder MR, Marriot D, Adams M. Stimulus secretion coupling in cultured chromaffin cells. Biochem Pharm. 56(1):163–167. 1987.
Article3. Albillos A, Artalejo AR, Lopez MG, Gandia L, Garcia AG, Carbone E. Calcium channel subtypes in cat chromaffin cells. J Physiol. 477:197–213. 1994.
Article4. Zimmermann H. Signalling via ATP in the nervous system. Trends Neurosci. 17:420–426. 1994.
Article5. Dunlap K, Fischbach GD. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurons. J Physiol. 317:519–535. 1981.6. Forscher P, Oxford GS. Modulation of calcium channels by norepinephrine in internally dialyzed avian sensory neurons. J Gen Physiol. 85:743–763. 1985.
Article7. Galvan M and Adams PR. Control of calcium current in rat sympathetic neurons by norepinephrine. Brain Res. 244:135–144. 1982.8. Dolphin AC and Scott RH. Calcium channel currents and their inhibition by (−)-baclofen in rat sensory neurons: modulation by guanine nucleotides. J Physiol. 386:1–17. 1987.9. Grassi F and Lux HD. Voltage-dependent GABA-induced modulation of calcium currents in chick sensory neurons. Neurosci Lett. 1015:113–119. 1989.10. Menon-Johansson AS, Berrow N, Dolphin AC. G(o) transduces GABAB-receptor modulation by N-type calcium channels in cultured dorsal root ganglion neurons. Pflugers Arch. 425:335–343. 1993.11. Harkins AB and Fox AP. Activation of purinergic receptors by ATP inhibits secretion in bovine adrenal chromaffin cells. Brain Res. 885:231–239. 2000.12. Carbone E, Carabelli V, Casetti T, Baldelli P, Hernandez-Guijo JM, Giusta L. G-protein and cAMP-dependent Lchannel gating mechanism: a manifold system to control calcium entry in neurosecretory cells. Pflugers Arch. 442(6):801–813. 2001.13. Diverse-Pierluissi M, Dunlap K, Westhead EW. Multiple actions of extracellular ATP on calcium currents in cultured bovine chromaffin cells. Proc Nat Acad Sci USA. 88:1261–1265. 1991.
Article14. Holz GG IV, Rane SG, Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 319:670–672. 1986.
Article15. Kajikawa Y, Saitoh N, Takahashi T. GTP-binding protein beta gamma subunits mediate presynaptic calcium current inhibition by GABA(B) receptor. Proc Nat Acad Sci USA. 98:8054–8058. 2001.16. Akaike A, Mine Y, Sasa M, Takaori S. Voltage and current clamp studies of M-and Nicotinic excitation of the rat chromaffin cells. J Pharmacol Exp Ther. 255:333–339. 1990.17. Hamil OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording from cells and cell free membrane patches. Pflugers Arch. 391:85–100. 1981.18. Marchetti C, Robello M. Guanosine-5'-O-(3-thiotriphosphate) modifies kinetics of voltage-dependent calcium current in chick sensory neurons. Biophys J. 56:1267–1272. 1989.
Article19. Artalejo CR, Rossie S, Perlman RL, Fox AP. Voltage dependent phosphorylation may recruit Ca2+ current facilitation in chromaffin cells. Nature. 358:63–66. 1992.20. Dolphin AC. Facilitation of Ca2+ current in excitable cells. Trends Neurosci. 19(1):35–43. 1996.
Article21. R. Eckert and J.E. Chad: Inactivation of calcium channels: Prog. Biophysi. Mol. Biol. 44:215–267. 1984.22. Powell AD, Teschemacher AG, Seward EP. P2Y purinoceptors inhibit exocytosis in adrenal chromaffin cells via modulation of voltage-operated calcium channels. J Neurosci. 20(2):606–616. 2000.
Article23. Dubyak GR, el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 265:C577–606. 1993.
Article24. Carabelli V, Hernández-Guijo JM, Baldelli P, Carbone E. Direct autocrine inhibition and cAMP-dependent potentiation of single L-type Ca2+ channels in bovine chromaffin cells. J Physiol. 532:(. (Pt 1):):. 73–90. 2001.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The characterization of the increase of membrane conductance after depolarization in single rat adrenal chromaffin cells
- The Effect of Lambert-Eaton Syndrome IgG on Calcium Current in Rat Chromaffin Cells
- Influence of glucocorticoids on cholinergic stimulation-induced catecholamine secretion from the rat adrenal medulla
- Pain-reducing Effect by Transplants of Isolated Xenogeneic Chromaffin Cells in Mouse
- Characteristics of Potassium Channel in the Isolated Rat Detrusor Muscle