Korean J Lab Med.
2006 Dec;26(6):424-430. 10.3343/kjlm.2006.26.6.424.
HBV DNA Quantitation Using Real-time PCR
- Affiliations
-
- 1Department of Internal Medicine, Pusan National University, School of Medicine, Busan, Korea.
- 2Department of Laboratory Medicine, Pusan National University, School of Medicine, Busan, Korea. hhkim@pusan.ac.kr
- 3Unit of Biomedical Informatics, Pusan National University, School of Medicine, Busan, Korea.
- KMID: 1889821
- DOI: http://doi.org/10.3343/kjlm.2006.26.6.424
Abstract
-
BACKGROUND: Accurate measurement of the concentration of hepatitis B virus (HBV) DNA in clinical samples is important for the appropriate treatment of patients and evaluation of their therapeutic responses. In addition, the concentration of HBV DNA in the serum of patients with chronic HBV infection has a very broad range. Real-time PCR is very sensitive and useful to detect HBV DNA in a wide range of concentrations. We designed new primers and probes for real-time PCR to detect HBV DNA.
METHODS
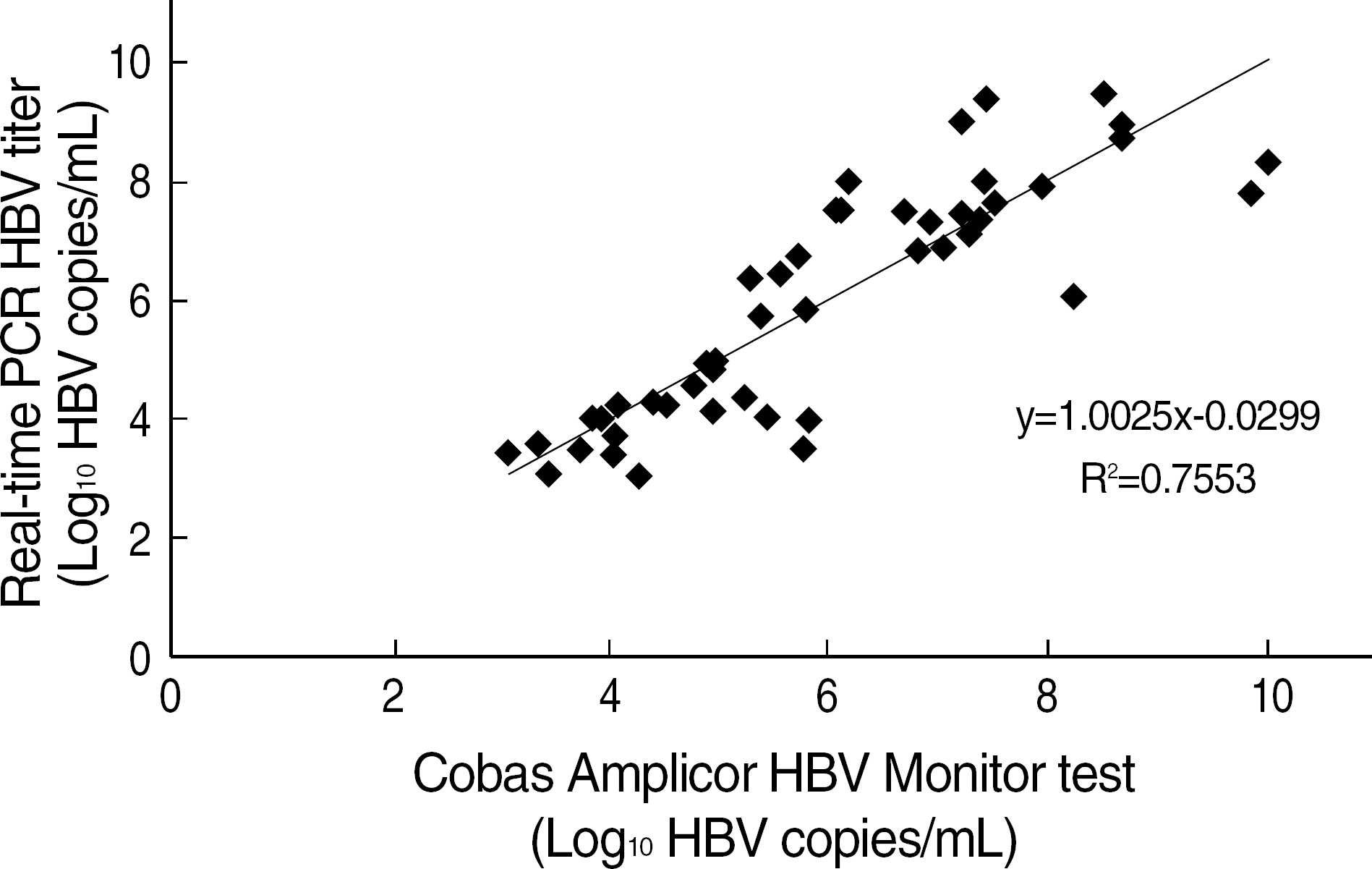
Primers and probes specific for HBV were designed. EUROHEP HBV DNA standards (NIBSC, Hertfordshire, UK) with the HBV DNA concentration of 7.0 x 10(4) copies/mL was used to determine the calibration curve and efficacy for the real-time PCR assay. Sensitivity, dynamic range, and precision were evaluated. The correlation between the real-time PCR and Cobas Amplicor HBV Monitor(TM) assay in the measurement of serum HBV DNA concentrations in 52 patients with chronic HBV infection was evaluated.
RESULTS
The sensitivity of the assay was approximately 6.08 x 10(2) copies/mL for HBV, and the quantitation was accurate and reproducible over a wide dynamic range from 6.1 x 0(2) to 6.5 x 10(9) copies/mL without any dilution of specimens. The assay showed low coefficients of variation of repeatability (3.7-24.9%) and reproducibility (7.8-24.7%). The results were found to correlate well with those obtained by Cobas Amplicor HBV Monitor(TM) kit.
CONCLUSIONS
We provide a new in-house method for the measurement of serum HBV DNA using real-time PCR, which enables us to detect HBV DNA rapidly, sensitively, and accurately.
Keyword
Figure
Reference
-
References
1. Hoofnagle JH, Shafritz DA, Popper H. Chronic type B hepatitis and the “healthy” HBsAg carrier state. Hepatology. 1987; 7:758–63.
Article2. Kaneko S, Miller RH, Di Bisceglie AM, Feinstone SM, Hoofnagle JH, Purcell RH. Detection of hepatitis B virus DNA in serum by polymerase chain reaction. Application for clinical diagnosis. Gastroenterology. 1990; 99:799–804.3. Hadziyannis SJ, Vassilopoulos D. Hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2001; 34:617–24.
Article4. Pawlotsky JM. Molecular diagnosis of viral hepatitis. Gastroenterology. 2002; 122:1554–68.
Article5. Saldanha J, Gerlich W, Lelie N, Dawson P, Heermann K, Heath A, et al. An international collaborative study to establish a World Health Organization international standard for hepatitis B virus DNA nucleic acid amplification techniques. Vox Sang. 2001; 80:63–71.
Article6. Aspinall S, Steele AD, Peenze I, Mphahlele MJ. Detection and quantitation of hepatitis B virus DNA: comparison of two commercial hybridization assays with polymerase chain reaction. J Viral Hepat. 1995; 2:107–11.
Article7. Barlet V, Cohard M, Thelu MA, Chaix MJ, Baccard C, Zarski JP, et al. Quantitative detection of hepatitis B virus DNA in serum using chemiluminescence: comparison with radioactive solution hybridization assay. J Virol Methods. 1994; 49:141–51.
Article8. Krajden M, Minor J, Cork L, Comanor L. Multi-measurement method comparison of three commercial hepatitis B virus DNA quantification assays. J Viral Hepat. 1998; 5:415–22.
Article9. Chan HL, Leung NW, Lau TC, Wong ML, Sung JJ. Comparison of three different sensitive assays for hepatitis B virus DNA in monitoring of responses to antiviral therapy. J Clin Microbiol. 2000; 38:3205–8.
Article10. Hendricks DA, Stowe BJ, Hoo BS, Kolberg J, Irvine BD, Neuwald PD, et al. Quantitation of HBV DNA in human serum using a branched DNA (bDNA) signal amplification assay. Am J Clin Pathol. 1995; 104:537–46.
Article11. Ho SK, Chan TM, Cheng IK, Lai KN. Comparison of the second-generation digene hybrid capture assay with the branched-DNA assay for measurement of hepatitis B virus DNA in serum. J Clin Microbiol. 1999; 37:2461–5.
Article12. Niesters HG, Krajden M, Cork L, de Medina M, Hill M, Fries E, et al. A multicenter study evaluation of the digene hybrid capture II signal amplification technique for detection of hepatitis B virus DNA in serum samples and testing of EUROHEP standards. J Clin Microbiol. 2000; 38:2150–5.
Article13. Loeb KR, Jerome KR, Goddard J, Huang M, Cent A, Corey L. High-throughput quantitative analysis of hepatitis B virus DNA in serum using the TaqMan fluorogenic detection system. Hepatology. 2000; 32:626–9.
Article14. Noborg U, Gusdal A, Horal P, Lindh M. Levels of viraemia in subjects with serological markers of past or chronic hepatitis B virus infection. Scand J Infect Dis. 2000; 32:249–52.15. Michalak TI, Pasquinelli C, Guilhot S, Chisari FV. Hepatitis B virus persistence after recovery from acute viral hepatitis. J Clin Invest. 1994; 93:230–9.
Article16. Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000-summary of a workshop. Gastroenterology. 2001; 120:1828–53.
Article17. Martinot-Peignoux M, Boyer N, Colombat M, Akremi R, Pham BN, Ollivier S, et al. Serum hepatitis B virus DNA levels and liver histology in inactive HBsAg carriers. J Hepatol. 2002; 36:543–6.
Article18. Heo J, Baik TH, Kim HH, Kim GH, Kang DH, Song GA, et al. Serum hepatitis B virus (HBV) DNA levels at different stages of clinical course in patients with chronic HBV infection in an endemic area. J Korean Med Sci. 2003; 18:686–90.19. Oh SH, Kim HH, Heo J, Cho M, Chang CH, Lee EY, et al. Serum hepatitis B virus DNA quantitive analysis using polymerase chain reaction in patients with chronic hepatitis B virus infection. Korean J Lab Med. 2003; 23:39–44.20. Abe A, Inoue K, Tanaka T, Kato J, Kajiyama N, Kawaguchi R, et al. Quantitation of hepatitis B virus genomic DNA by real-time detection PCR. J Clin Microbiol. 1999; 37:2899–903.
Article21. Pas SD, Fries E, De Man RA, Osterhaus AD, Niesters HG. Development of a quantitative real-time detection assay for hepatitis B virus DNA and comparison with two commercial assays. J Clin Microbiol. 2000; 38:2897–901.
Article22. Weinberger KM, Wiedenmann E, Bohm S, Jilg W. Sensitive and accurate quantitation of hepatitis B virus DNA using a kinetic fluorescence detection system (TaqMan PCR). J Virol Methods. 2000; 85:75–82.
Article23. Zanella I, Rossini A, Domenighini D, Albertini A, Cariani E. Quantitative analysis of hepatitis B virus DNA by real-lime amplification. Eur J Clin Microbiol Infect Dis. 2002; 21:22–6.24. Weiss J, Wu H, Farrenkopf B, Schultz T, Song G, Shah S, et al. Real time TaqMan PCR detection and quantitation of HBV genotypes A-G with the use of an internal quantitation standard. J Clin Virol. 2004; 30:86–93.
Article25. Pawlotsky JM, Bastie A, Hezode C, Lonjon I, Darthuy F, Remire J, et al. Routine detection and quantification of hepatitis B virus DNA in clinical laboratories: performance of three commercial assays. J Virol Methods. 2000; 85:11–21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Evaluation of HBeAg Quantitation Using Enzyme Immunoassay in the Follow-Up of Patients with Chronic Hepatitis B
- Evaluation of Artus HBV LC PCR kit using SLAN Real-time PCR
- Comparison of Hybrid Capture System, Hybrid Capture II and Quantiplex HBV DNA Assay for Quantitation of Hepatitis B Virus DNA
- Comparison of Quantitation of Cytomegalovirus DNA by Real-Time PCR in Whole Blood with the Cytomegalovirus Antigenemia Assay
- The outcome of perinatal prophylaxis for HBeAg positive mothers according to the maternal HBV-DNA levels at the delivery time