Ann Lab Med.
2015 Jan;35(1):99-104. 10.3343/alm.2015.35.1.99.
Comparison of Quantitation of Cytomegalovirus DNA by Real-Time PCR in Whole Blood with the Cytomegalovirus Antigenemia Assay
- Affiliations
-
- 1Department of Laboratory Medicine, College of Medicine, Korea University, Seoul, Korea. eqcho1ku@korea.ac.kr
- KMID: 2363155
- DOI: http://doi.org/10.3343/alm.2015.35.1.99
Abstract
- BACKGROUND
Quantitation of cytomegalovirus (CMV) DNA using real-time PCR has been utilized for monitoring CMV infection. However, the CMV antigenemia assay is still the 'gold standard' assay. There are only a few studies in Korea that compared the efficacy of use of real-time PCR for quantitation of CMV DNA in whole blood with the antigenemia assay, and most of these studies have been limited to transplant recipients. METHOD: 479 whole blood samples from 79 patients, falling under different disease groups, were tested by real-time CMV DNA PCR using the Q-CMV real-time complete kit (Nanogen Advanced Diagnostic S.r.L., Italy) and CMV antigenemia assay (CINA Kit, ArgeneBiosoft, France), and the results were compared. Repeatedly tested patients were selected and their charts were reviewed for ganciclovir therapy.
RESULTS
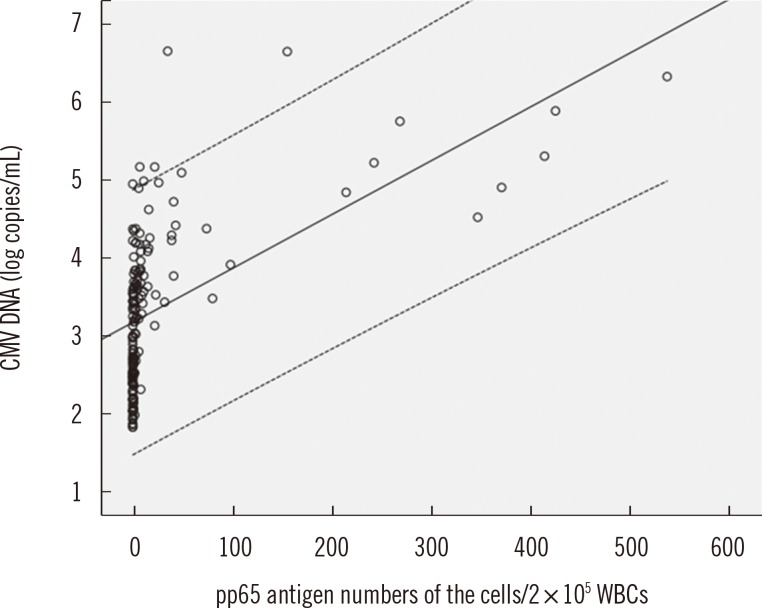
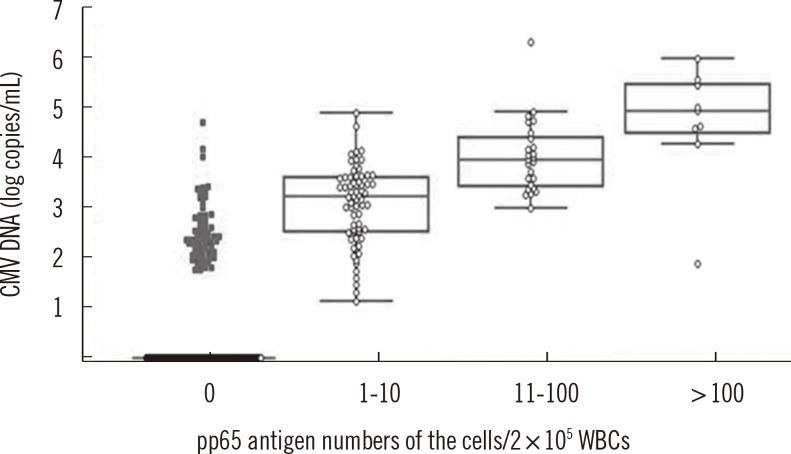
The concordance rate of the two assays was 86.4% (Cohen's kappa coefficient value=0.659). Quantitative correlation between the two assays was a moderate (r=0.5504, P<0.0001). Among 20 patients tested repeatedly with the two assays, 13 patients were transplant recipients and treated with ganciclovir. Before treatment, CMV was detected earlier by real-time CMV DNA PCR than the antigenemia assay, with a median difference of 8 days. After treatment, the antigenemia assay achieved negative results earlier than real-time CMV DNA PCR with a median difference of 10.5 days.
CONCLUSIONS
Q-CMV real-time complete kit is a useful tool for early detection of CMV infection in whole blood samples in transplant recipients.
Keyword
MeSH Terms
-
Antiviral Agents/therapeutic use
Cytomegalovirus/*genetics
Cytomegalovirus Infections/drug therapy/pathology/virology
DNA, Viral/*blood/metabolism
Ganciclovir/therapeutic use
Humans
*Immunoassay
Organ Transplantation
Phosphoproteins/genetics/immunology/*metabolism
*Real-Time Polymerase Chain Reaction
Viral Matrix Proteins/genetics/immunology/*metabolism
Virology/*methods
Antiviral Agents
DNA, Viral
Ganciclovir
Phosphoproteins
Viral Matrix Proteins
Figure
Cited by 1 articles
-
Laboratory diagnostic testing for cytomegalovirus infection in solid organ transplant patients
Hyeyoung Lee, Eun-Jee Oh
Korean J Transplant. 2022;36(1):15-28. doi: 10.4285/kjt.22.0001.
Reference
-
1. Paya CV. Prevention of cytomegalovirus disease in recipients of solid-organ transplants. Clin Infect Dis. 2001; 32:596–603. PMID: 11181123.2. Boeckh M, Boivin G. Quantitation of cytomegalovirus: methodologic aspects and clinical applications. Clin Microbiol Rev. 1998; 11:533–554. PMID: 9665982.
Article3. Allice T, Cerutti F, Pittaluga F, Varetto S, Franchello A, Salizzoni M, et al. Evaluation of a novel real-time PCR system for cytomegalovirus DNA quantitation on whole blood and correlation with pp65-antigen test in guiding pre-emptive antiviral treatment. J Virol Methods. 2008; 148:9–16. PMID: 18045702.
Article4. Guiver M, Fox AJ, Mutton K, Mogulkoc N, Egan J. Evaluation of CMV viral load using TaqMan CMV quantitative PCR and comparison with CMV antigenemia in heart and lung transplant recipients. Transplantation. 2001; 71:1609–1615. PMID: 11435973.
Article5. Camargo LF, Uip DE, Simpson AA, Caballero O, Stolf NA, Vilas-Boas LS, et al. Comparison between antigenemia and a quantitative-competitive polymerase chain reaction for the diagnosis of cytomegalovirus infection after heart transplantation. Transplantation. 2001; 71:412–417. PMID: 11233903.6. Heid CA, Stevens J, Livak KJ, Williams PM. Realtime quantitative PCR. Genome Res. 1996; 6:986–994. PMID: 8908518.7. Choi SM, Lee DG, Lim J, Park SH, Choi JH, Yoo JH, et al. Comparison of quantitative cytomegalovirus real-time PCR in whole blood and pp65 antigenemia assay: clinical utility of CMV real-time PCR in hematopoietic stem cell transplant recipients. J Korean Med Sci. 2009; 24:571–578. PMID: 19654935.
Article8. Marchetti S, Santangelo R, Manzara S, D'onghia S, Fadda G, Cattani P. Comparison of real-time PCR and pp65 antigen assays for monitoring the development of Cytomegalovirus disease in recipients of solid organ and bone marrow transplants. New Microbiol. 2011; 34:157–164. PMID: 21617827.9. Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002; 34:1094–1097. PMID: 11914998.
Article10. Paya CV, Wilson JA, Espy MJ, Sia IG, DeBernardi MJ, Smith TF, et al. Preemptive use of oral ganciclovir to prevent cytomegalovirus infection in liver transplant patients: a randomized, placebo-controlled trial. J Infect Dis. 2002; 185:854–860. PMID: 11920308.
Article11. Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013; 96:333–360. PMID: 23896556.
Article12. Griffiths PD, Whitley RJ, editors. Recommendations from the International Herpes Management Forum (IHMF) Managements Strategies Workshop and 8th Annual Meeting of the IHMF. The challenge of CMV infection and disease in transplantation. UK: Cambridge Medical Publications;2000.13. Lilleri D, Baldanti F, Gatti M, Rovida F, Dossena L, De Grazia S, et al. Clinically-based determination of safe DNAemia cutoff levels for preemptive therapy or human cytomegalovirus infections in solid organ and hematopoietic stem cell transplant recipients. J Med Virol. 2004; 73:412–418. PMID: 15170637.
Article14. Razonable RR, Brown RA, Wilson J, Groettum C, Kremers W, Espy M, et al. The clinical use of various blood compartments for cytomegalovirus (CMV) DNA quantitation in transplant recipients with CMV disease. Transplantation. 2002; 73:968–973. PMID: 11923702.
Article15. Von Müller L, Hampl W, Hinz J, Meisel H, Reip A, Engelmann E, et al. High variability between results of different in-house tests for cytomegalovirus (CMV) monitoring and a standardized quantitative plasma CMV PCR assay. J Clin Microbiol. 2002; 40:2285–2287. PMID: 12037112.
Article16. Heo WB, Won DI, Kim YL, Kim MH, Oh HB, Suh JS. Evaluation of Biosewoom Real-Q Cytomegalovirus Quantification kit for Cytomegalovirus viral load measure. Korean J Lab Med. 2007; 27:298–304. PMID: 18094592.17. Li H, Dummer JS, Estes WR, Meng S, Wright PF, Tang YW. Measurement of human cytomegalovirus loads by quantitative real-time PCR for monitoring clinical intervention in transplant recipients. J Clin Microbiol. 2003; 41:187–191. PMID: 12517846.
Article18. Kalpoe JS, Kroes AC, de Jong MD, Schinkel J, de Brouwer CS, Beersma MF, et al. Validation of clinical application of cytomegalovirus plasma DNA load measurement and definition of treatment criteria by analysis of correlation to antigen detection. J Clin Microbiol. 2004; 42:1498–1504. PMID: 15070995.
Article19. Mhiri L, Kaabi B, Houimel M, Arrouji Z, Slim A. Comparison of pp65 antigenemia, quantitative PCR and DNA hybrid capture for detection of cytomegalovirus in transplant recipients and AIDS patients. J Virol Methods. 2007; 143:23–28. PMID: 17336402.
Article20. Ghaffari SH, Obeidi N, Dehghan M, Alimoghaddam K, Gharehbaghian A, Ghavamzadeh A. Monitoring of cytomegalovirus reactivation in bone marrow transplant recipients by real-time PCR. Pathol Oncol Res. 2008; 14:399–409. PMID: 18392952.
Article21. Pillet S, Roblin X, Cornillon J, Mariat C, Pozzetto B. Quantification of cytomegalovirus viral load. Expert Rev Anti Infect Ther. 2014; 12:193–210. PMID: 24341395.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- When is an Assay of Cytomegalovirus Antigenemia Useful in Detecting Cytomegalovirus Colitis?
- Comparison of Real-time PCR Methods and pp65 Antigenemia Assay to Detect Cytomegalovirus Reactivation in Hematopoietic Stem Cell Transplantation
- Performance Evaluation of the Real-Q Cytomegalovirus (CMV) Quantification Kit Using Two Real-Time PCR Systems for Quantifying CMV DNA in Whole Blood
- Comparison of Cytomegalovirus Antigenemia with Roche Amplicor CMV test for Detection of Cytomegalovirus Infection in Bone Marrow Transplant Recipients
- Comparison of Polymerase Chain Reaction Method and CMV Antigenemia Assay for Diagnosis of Cytomegalovirus Infection in Transplanted Patients