Ann Surg Treat Res.
2014 May;86(5):256-263. 10.4174/astr.2014.86.5.256.
High pretransplant HBV level predicts HBV reactivation after kidney transplantation in HBV infected recipients
- Affiliations
-
- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. kmhyj111@skku.edu
- 2Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1889663
- DOI: http://doi.org/10.4174/astr.2014.86.5.256
Abstract
- PURPOSE
HBsAg-positive kidney recipients are at increased risk for mortality and graft failure. The aims of this study were to identify the outcomes of HBsAg-positive recipients who received preemptive antiviral agents after successful kidney transplantation and to analyze risk factors for HBV reactivation.
METHODS
We retrospectively reviewed the medical records of 944 patients performed kidney transplantation between 1999 and 2010.
RESULTS
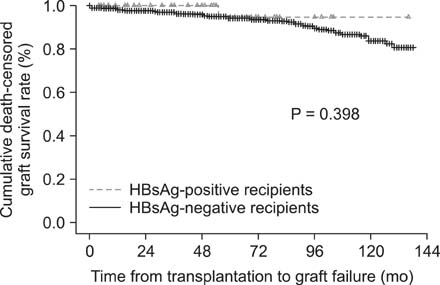
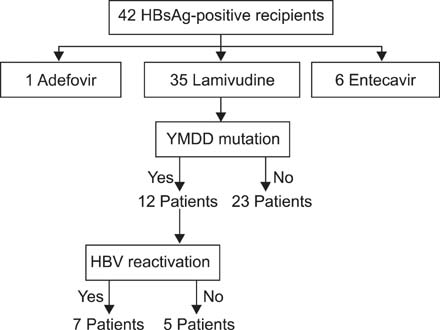
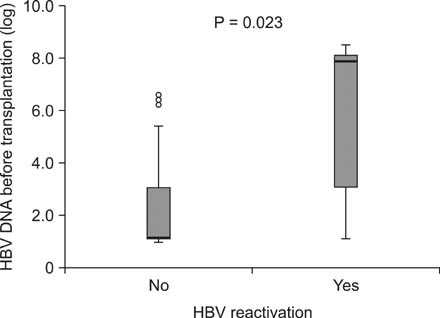
HBsAg-negative recipients were 902 patients and HBsAg-positive recipients, 42. Among HBsAg-positive recipients, HBV reactivation was detected in 7 patients and well controlled by switch or combination therapy. Graft failure developed in only one patient due to chronic rejection regardless of HBV reactivation but no deaths occurred. All patients were alive at the end of follow-up and none developed end-stage liver disease or hepatocellular carcinoma. There was statistically significant difference in graft survival between HBsAg-positive recipients and HBsAg-negative. Multivariate analysis identified increased HBV DNA levels (>5 x 10(4) IU/mL) in the HBsAg-positive kidney transplant recipients as a risk factor for HBV reactivation (P = 0.007).
CONCLUSION
Effective viral suppression with antiviral agents in HBsAg-positive renal transplant recipients improves patient outcome and allograft survival. Antiviral therapy may be especially beneficial in patients with high HBV DNA levels prior to transplantation.
MeSH Terms
Figure
Cited by 1 articles
-
Hepatocellular carcinoma and cancer-related mortality after kidney transplantation with rituximab treatment
Hayoung Lee, Young Hoon Kim, Seong Jun Lim, Youngmin Ko, Sung Shin, Joo Hee Jung, Chung Hee Baek, Hyosang Kim, Su-Kil Park, Hyunwook Kwon
Ann Surg Treat Res. 2022;102(1):55-63. doi: 10.4174/astr.2022.102.1.55.
Reference
-
1. Korean Association for the Study of the Liver. KASL Clinical Practice Guidelines: management of chronic hepatitis B. Clin Mol Hepatol. 2012; 18:109–162.2. Kalia H, Fabrizi F, Martin P. Hepatitis B virus and renal transplantation. Transplant Rev (Orlando). 2011; 25:102–109.3. Ivanyi B. A primer on recurrent and de novo glomerulonephritis in renal allografts. Nat Clin Pract Nephrol. 2008; 4:446–457.4. Aroldi A, Lampertico P, Montagnino G, Passerini P, Villa M, Campise MR, et al. Natural history of hepatitis B and C in renal allograft recipients. Transplantation. 2005; 79:1132–1136.5. Fabrizi F, Martin P, Dixit V, Kanwal F, Dulai G. HBsAg seropositive status and survival after renal transplantation: meta-analysis of observational studies. Am J Transplant. 2005; 5:2913–2921.6. Perrillo RP. Hepatitis B and renal transplantation: securing the sword of damocles. Hepatology. 2002; 36:1041–1045.7. Mathurin P, Mouquet C, Poynard T, Sylla C, Benalia H, Fretz C, et al. Impact of hepatitis B and C virus on kidney transplantation outcome. Hepatology. 1999; 29:257–263.8. Ahn HJ, Kim MS, Kim YS, Kim SI, Huh KH, Ju MK, et al. Clinical outcome of renal transplantation in patients with positive pre-transplant hepatitis B surface antigen. J Med Virol. 2007; 79:1655–1663.9. Degos F, Lugassy C, Degott C, Debure A, Carnot F, Theirs V, et al. Hepatitis B virus and hepatitis B-related viral infection in renal transplant recipients: a prospective study of 90 patients. Gastroenterology. 1988; 94:151–156.10. Parfrey PS, Forbes RD, Hutchinson TA, Beaudoin JG, Dauphinee WD, Hollomby DJ, et al. The clinical and pathological course of hepatitis B liver disease in renal transplant recipients. Transplantation. 1984; 37:461–466.11. Tur-Kaspa R, Shaul Y, Moore DD, Burk RD, Okret S, Poellinger L, et al. The glucocorticoid receptor recognizes a specific nucleotide sequence in hepatitis B virus DNA causing increased activity of the HBV enhancer. Virology. 1988; 167:630–633.12. Cheng AL, Hsiung CA, Su IJ, Chen PJ, Chang MC, Tsao CJ, et al. Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology. 2003; 37:1320–1328.13. Therret E, Pol S, Legendre C, Gagnadoux MF, Cavalcanti R, Kreis H. Low-dose recombinant leukocyte interferon-alpha treatment of hepatitis C viral infection in renal transplant recipients: a pilot study. Transplantation. 1994; 58:625–628.14. Allen MI, Deslauriers M, Andrews CW, Tipples GA, Walters KA, Tyrrell DL, et al. Lamivudine Clinical Investigation Group. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Hepatology. 1998; 27:1670–1677.15. Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, et al. Asia Hepatitis Lamivudine Study Group. A one-year trial of lamivudine for chronic hepatitis B. N Engl J Med. 1998; 339:61–68.16. Lok AS, Lai CL, Leung N, Yao GB, Cui ZY, Schiff ER, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology. 2003; 125:1714–1722.17. European Association for the Study of the Liver. EASL clinical practice guidelines. Management of chronic hepatitis B. Gastroenterol Clin Biol. 2009; 33:539–554.18. Marzano A, Gaia S, Ghisetti V, Carenzi S, Premoli A, Debernardi-Venon W, et al. Viral load at the time of liver transplantation and risk of hepatitis B virus recurrence. Liver Transpl. 2005; 11:402–409.19. Beckebaum S, Sotiropoulos GC, Klein CG, Broelsch CE, Saner F, Paul A, et al. Predictive factors of outcome in patients transplanted for hepatitis B. Transplantation. 2009; 87:872–881.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- De novo hepatitis B virus infection after liver transplantation from anti-hepatitis B core antibody positive donor: a 20-year experience at a single center
- HBV reactivation in a HBsAg-negative patient with multiple myeloma treated with prednisolone maintenance therapy after autologous HSCT
- Prevention of Hepatitis B reactivation in the setting of immunosuppression
- Hepatitis B Virus Reactivation after Partial Hepatic Irradiation Alone: A Case Report
- Hepatitis B virus reactivation in a liver transplant recipient using hepatitis B immunoglobulin plus antiviral drug