Korean Circ J.
2014 Jan;44(1):1-9. 10.4070/kcj.2014.44.1.1.
Cardiovascular Molecular Imaging with Contrast Ultrasound: Principles and Applications
- Affiliations
-
- 1Knight Cardiovascular Institute, Oregon Health & Science University, Portland, OR, USA. lindnerj@ohsu.edu
- KMID: 1859235
- DOI: http://doi.org/10.4070/kcj.2014.44.1.1
Abstract
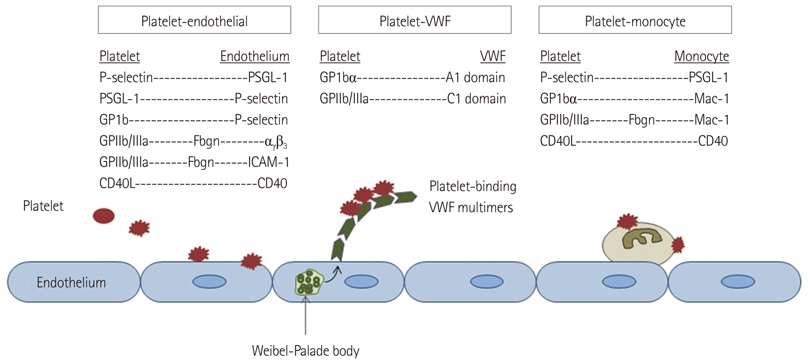
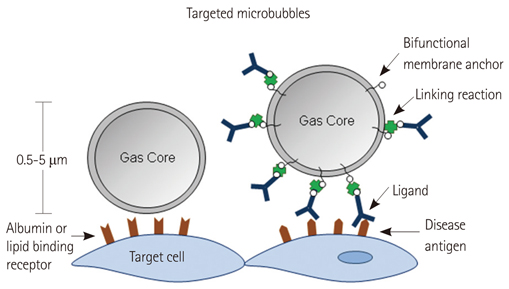
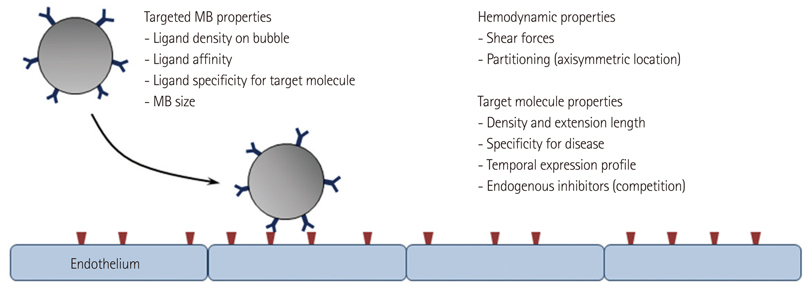
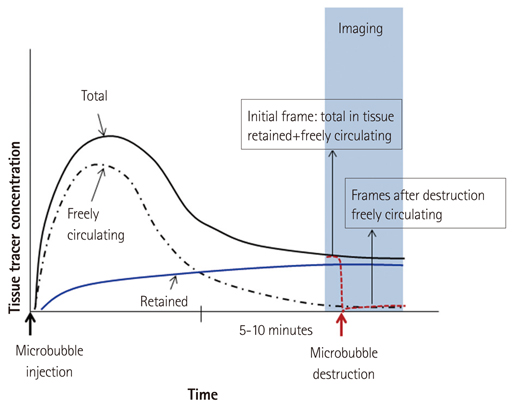
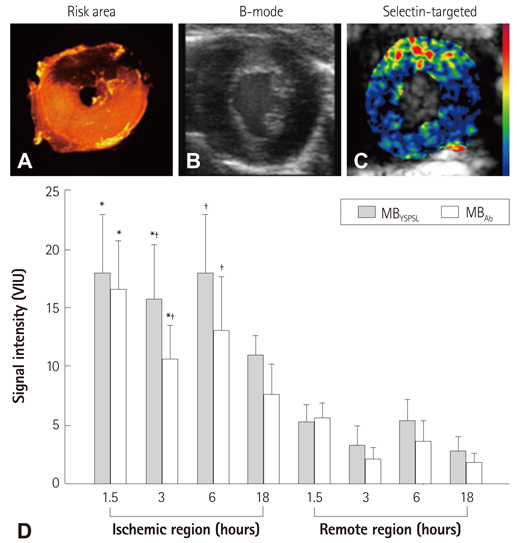
- Methods for imaging the molecular or cellular profile of tissue are being developed for all forms of non-invasive cardiovascular imaging. It is thought that these technologies will potentially improve patient outcomes by allowing diagnosis of disease at an early-stage, monitoring disease progression, providing important information on patient risk, and for tailoring therapy to the molecular basis of disease. Molecular imaging is also already assuming an important role in science by providing a better understanding of the molecular basis of cardiovascular pathology, for assessing response to new therapies, and for rapidly optimizing new or established therapies. Ultrasound-based molecular imaging is one of these new approaches. Contrast-enhanced ultrasound molecular imaging relies on the detection of novel site-targeted microbubbles (MB) or other acoustically active particles which are administered by intravenous injection, circulate throughout the vascular compartment, and are then retained and imaged within regions of disease by ligand-directed binding. The technique is thought to be advantageous in practical terms of cost, time, and ease of use. The aim of this review is to discuss the molecular participants of cardiovascular disease that have been targeted for ultrasound imaging, general features of site-targeted MB, imaging protocols, and potential roles of ultrasound molecular imaging in cardiovascular research and clinical medicine.
Keyword
MeSH Terms
Figure
Reference
-
1. Sinusas AJ, Bengel F, Nahrendorf M, et al. Multimodality cardiovascular molecular imaging, part I. Circ Cardiovasc Imaging. 2008; 1:244–256.2. Inaba Y, Lindner JR. Molecular imaging of disease with targeted contrast ultrasound imaging. Transl Res. 2012; 159:140–148.3. Gessner R, Dayton PA. Advances in molecular imaging with ultrasound. Mol Imaging. 2010; 9:117–127.4. Lindner JR, Song J, Jayaweera AR, Sklenar J, Kaul S. Microvascular rheology of Definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr. 2002; 15:396–403.5. Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999; 340:115–126.6. Cines DB, Pollak ES, Buck CA, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998; 91:3527–3561.7. Springer TA. Adhesion receptors of the immune system. Nature. 1990; 346:425–434.8. Ley K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc Res. 1996; 32:733–742.9. Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003; 9:263–268.10. Hamilton AJ, Huang SL, Warnick D, et al. Intravascular ultrasound molecular imaging of atheroma components in vivo. J Am Coll Cardiol. 2004; 43:453–460.11. Lanza GM, Wallace KD, Scott MJ, et al. A novel site-targeted ultrasonic contrast agent with broad biomedical application. Circulation. 1996; 94:3334–3340.12. Lanza GM, Abendschein DR, Hall CS, et al. In vivo molecular imaging of stretch-induced tissue factor in carotid arteries with ligand-targeted nanoparticles. J Am Soc Echocardiogr. 2000; 13:608–614.13. Bassenge E. Antiplatelet effects of endothelium-derived relaxing factor and nitric oxide donors. Eur Heart J. 1991; 12:Suppl E. 12–15.14. Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004; 109:23 Suppl 1. III27–III32.15. Alonso A, Della Martina A, Stroick M, et al. Molecular imaging of human thrombus with novel abciximab immunobubbles and ultrasound. Stroke. 2007; 38:1508–1514.16. Wang X, Hagemeyer CE, Hohmann JD, et al. Novel single-chain antibody-targeted microbubbles for molecular ultrasound imaging of thrombosis: validation of a unique noninvasive method for rapid and sensitive detection of thrombi and monitoring of success or failure of thrombolysis in mice. Circulation. 2012; 125:3117–3126.17. Ruggeri ZM. Von Willebrand factor, platelets and endothelial cell interactions. J Thromb Haemost. 2003; 1:1335–1342.18. Liu Y, Davidson BP, Yue Q, et al. Molecular imaging of inflammation and platelet adhesion in advanced atherosclerosis effects of antioxidant therapy with NADPH oxidase inhibition. Circ Cardiovasc Imaging. 2013; 6:74–82.19. McCarty OJ, Conley RB, Shentu W, et al. Molecular imaging of activated von Willebrand factor to detect high-risk atherosclerotic phenotype. JACC Cardiovasc Imaging. 2010; 3:947–955.20. Leong-Poi H, Christiansen J, Klibanov AL, Kaul S, Lindner JR. Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to alpha(v)-integrins. Circulation. 2003; 107:455–460.21. Leong-Poi H, Christiansen J, Heppner P, et al. Assessment of endogenous and therapeutic arteriogenesis by contrast ultrasound molecular imaging of integrin expression. Circulation. 2005; 111:3248–3254.22. Dayton PA, Pearson D, Clark J, et al. Ultrasonic analysis of peptide- and antibody-targeted microbubble contrast agents for molecular imaging of alphavbeta3-expressing cells. Mol Imaging. 2004; 3:125–134.23. Palmowski M, Huppert J, Ladewig G, et al. Molecular profiling of angiogenesis with targeted ultrasound imaging: early assessment of antiangiogenic therapy effects. Mol Cancer Ther. 2008; 7:101–109.24. Behm CZ, Kaufmann BA, Carr C, et al. Molecular imaging of endothelial vascular cell adhesion molecule-1 expression and inflammatory cell recruitment during vasculogenesis and ischemia-mediated arteriogenesis. Circulation. 2008; 117:2902–2911.25. Ryu JC, Davidson BP, Xie A, et al. Molecular imaging of the paracrine proangiogenic effects of progenitor cell therapy in limb ischemia. Circulation. 2013; 127:710–719.26. Winter PM, Morawski AM, Caruthers SD, et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation. 2003; 108:2270–2274.27. Lindner JR. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov. 2004; 3:527–532.28. Lindner JR, Coggins MP, Kaul S, Klibanov AL, Brandenburger GH, Ley K. Microbubble persistence in the microcirculation during ischemia/reperfusion and inflammation is caused by integrin- and complement-mediated adherence to activated leukocytes. Circulation. 2000; 101:668–675.29. Lindner JR, Dayton PA, Coggins MP, et al. Noninvasive imaging of inflammation by ultrasound detection of phagocytosed microbubbles. Circulation. 2000; 102:531–538.30. Lindner JR, Song J, Xu F, et al. Noninvasive ultrasound imaging of inflammation using microbubbles targeted to activated leukocytes. Circulation. 2000; 102:2745–2750.31. Behm CZ, Lindner JR. Cellular and molecular imaging with targeted contrast ultrasound. Ultrasound Q. 2006; 22:67–72.32. Quillard T, Libby P. Molecular imaging of atherosclerosis for improving diagnostic and therapeutic development. Circ Res. 2012; 111:231–244.33. Kaufmann BA, Carr CL, Belcik JT, et al. Molecular imaging of the initial inflammatory response in atherosclerosis: implications for early detection of disease. Arterioscler Thromb Vasc Biol. 2010; 30:54–59.34. Villanueva FS, Jankowski RJ, Klibanov S, et al. Microbubbles targeted to intercellular adhesion molecule-1 bind to activated coronary artery endothelial cells. Circulation. 1998; 98:1–5.35. Kaufmann BA, Sanders JM, Davis C, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007; 116:276–284.36. Chadderdon SM, Belcik JT, Bader L, et al. Pro-Inflammatory Endothelial Activation Detected by Molecular Imaging in Obese Non-Human Primates Coincides with the Onset of Insulin Resistance and Progressively Increases with Duration of Insulin Resistance. Circulation. 2013; [Epub ahead of print].37. Anderson DR, Tsutsui JM, Xie F, Radio SJ, Porter TR. The role of complement in the adherence of microbubbles to dysfunctional arterial endothelium and atherosclerotic plaque. Cardiovasc Res. 2007; 73:597–606.38. Xie F, Lof J, Matsunaga T, Zutshi R, Porter TR. Diagnostic ultrasound combined with glycoprotein IIb/IIIa-targeted microbubbles improves microvascular recovery after acute coronary thrombotic occlusions. Circulation. 2009; 119:1378–1385.39. Davidson BP, Lindner JR. Future applications of contrast echocardiography. Heart. 2012; 98:246–253.40. Christiansen JP, French BA, Klibanov AL, Kaul S, Lindner JR. Targeted tissue transfection with ultrasound destruction of plasmid-bearing cationic microbubbles. Ultrasound Med Biol. 2003; 29:1759–1767.41. Davidson BP, Kaufmann BA, Belcik JT, Xie A, Qi Y, Lindner JR. Detection of antecedent myocardial ischemia with multiselectin molecular imaging. J Am Coll Cardiol. 2012; 60:1690–1697.42. Villanueva FS, Lu E, Bowry S, et al. Myocardial ischemic memory imaging with molecular echocardiography. Circulation. 2007; 115:345–352.43. Leong-Poi H. Molecular imaging using contrast-enhanced ultrasound: evaluation of angiogenesis and cell therapy. Cardiovasc Res. 2009; 84:190–200.44. Sang QX. Complex role of matrix metalloproteinases in angiogenesis. Cell Res. 1998; 8:171–177.45. Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994; 264:569–571.46. Pysz MA, Guracar I, Tian L, Willmann JK. Fast microbubble dwell-time based ultrasonic molecular imaging approach for quantification and monitoring of angiogenesis in cancer. Quant Imaging Med Surg. 2012; 2:68–80.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Future Applications of Contrast Ultrasound
- A Primer on the Methods and Applications for Contrast Echocardiography in Clinical Imaging
- Photoacoustic microscopy: principles and biomedical applications
- Ultrasound Contrast Agent
- Microbubbles used for contrast enhanced ultrasound and theragnosis: a review of principles to applications