J Cardiovasc Ultrasound.
2011 Sep;19(3):107-114. 10.4250/jcu.2011.19.3.107.
Future Applications of Contrast Ultrasound
- Affiliations
-
- 1Cardiovascular Division, Oregon Health & Science University, Portland, OR, USA. weik@ohsu.edu
- KMID: 1980363
- DOI: http://doi.org/10.4250/jcu.2011.19.3.107
Abstract
- Contrast agents are currently used during echocardiography for enhancement of structure and function, as well as for perfusion imaging. The next frontiers in contrast ultrasonography are targeted imaging, and using microbubbles for therapeutic purposes. Targeted imaging is the detection of specific components of cardiovascular disease in vivo, with microbubbles which may non-specifically attach to diseased endothelial cells, or with microbubbles which have been specifically designed to detect a pathologic process. Therapeutic applications of contrast ultrasonography include the use of microbubbles to enhance delivery of agents (like drugs, genes, growth factors, etc.) to the endothelium or perivascular cells. This review will discuss differences in contrast agents used for current applications versus targeted imaging, technical considerations required to achieve site-specific imaging, and potential applications of this technology. The potential for contrast ultrasonography to enhance drug and gene delivery to tissue will also be discussed.
Keyword
MeSH Terms
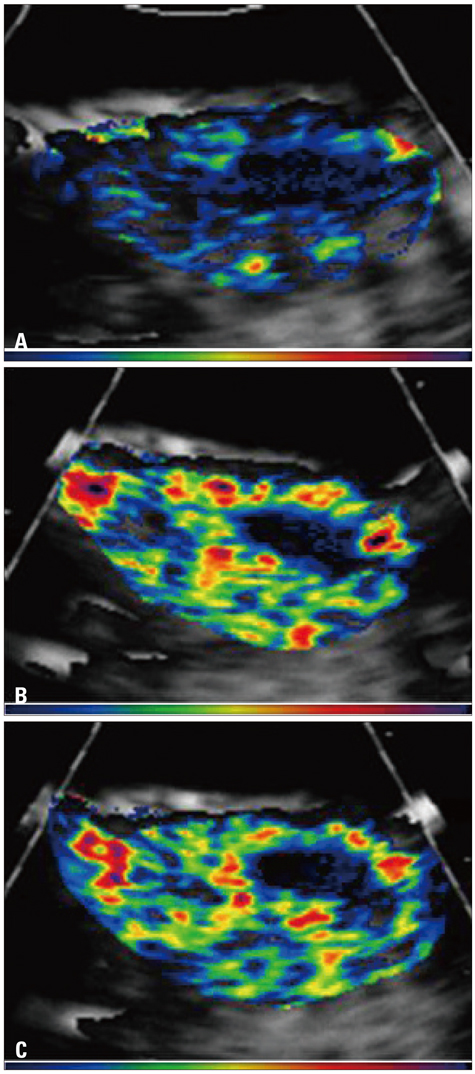
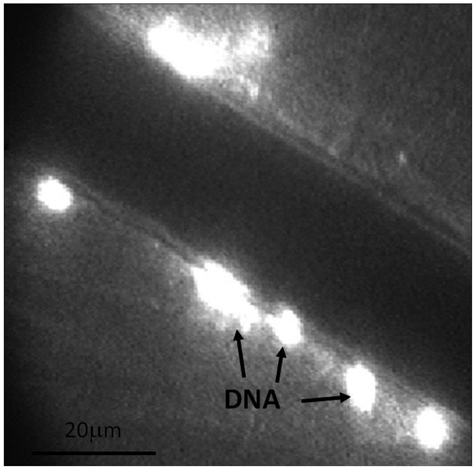
Figure
Reference
-
1. de Jong N, Frinking PJ, Bouakaz A, Goorden M, Schourmans T, Jingping X, Mastik F. Optical imaging of contrast agent microbubbles in an ultrasound field with a 100-MHz camera. Ultrasound Med Biol. 2000. 26:487–492.
Article2. Dayton PA, Morgan KE, Klibanov AL, Brandenburger GH, Ferrara KW. Optical and acoustical observations of the effects of ultrasound on contrast agents. IEEE Trans Ultrason Ferroelectr Freq Control. 1999. 46:220–232.
Article3. Keller MW, Glasheen W, Teja K, Gear A, Kaul S. Myocardial contrast echocardiography without significant hemodynamic effects or reactive hyperemia: a major advantage in the imaging of regional myocardial perfusion. J Am Coll Cardiol. 1988. 12:1039–1047.
Article4. Keller MW, Segal SS, Kaul S, Duling B. The behavior of sonicated albumin microbubbles within the microcirculation: a basis for their use during myocardial contrast echocardiography. Circ Res. 1989. 65:458–467.
Article5. Jayaweera AR, Edwards N, Glasheen WP, Villanueva FS, Abbott RD, Kaul S. In vivo myocardial kinetics of air-filled albumin microbubbles during myocardial contrast echocardiography. Comparison with radiolabeled red blood cells. Circ Res. 1994. 74:1157–1165.
Article6. Skyba DM, Camarano G, Goodman NC, Price RJ, Skalak TC, Kaul S. Hemodynamic characteristics, myocardial kinetics and microvascular rheology of FS-069, a second-generation echocardiographic contrast agent capable of producing myocardial opacification from a venous injection. J Am Coll Cardiol. 1996. 28:1292–1300.
Article7. Moore CA, Smucker ML, Kaul S. Myocardial contrast echocardiography in humans: I. Safety--a comparison with routine coronary arteriography. J Am Coll Cardiol. 1986. 8:1066–1072.
Article8. Villanueva FS, Jankowski RJ, Manaugh C, Wagner WR. Albumin microbubble adherence to human coronary endothelium: implications for assessment of endothelial function using myocardial contrast echocardiography. J Am Coll Cardiol. 1997. 30:689–693.
Article9. Fisher NG, Christiansen JP, Klibanov A, Taylor RP, Kaul S, Lindner JR. Influence of microbubble surface charge on capillary transit and myocardial contrast enhancement. J Am Coll Cardiol. 2002. 40:811–819.
Article10. Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990. 268:235–237.
Article11. Torchilin VP, Omelyanenko VG, Papisov MI, Bogdanov AA Jr, Trubetskoy VS, Herron JN, Gentry CA. Poly(ethylene glycol) on the liposome surface: on the mechanism of polymer-coated liposome longevity. Biochim Biophys Acta. 1994. 1195:11–20.
Article12. Jayaweera AR, Matthew TL, Sklenar J, Spotnitz WD, Watson DD, Kaul S. Method for the quantitation of myocardial perfusion during myocardial contrast two-dimensional echocardiography. J Am Soc Echocardiogr. 1990. 3:91–98.
Article13. Jayaweera AR, Ismail S, Kaul S. Attenuation deforms time-intensity curves during contrast echocardiography: implications for assessment of mean transit rates. J Am Soc Echocardiogr. 1994. 7:590–597.
Article14. Lindner JR, Coggins MP, Kaul S, Klibanov AL, Brandenburger GH, Ley K. Microbubble persistence in the microcirculation during ischemia/reperfusion and inflammation is caused by integrin- and complement-mediated adherence to activated leukocytes. Circulation. 2000. 101:668–675.
Article15. Lindner JR, Chomas JE, Phillips PJ. Cellular and molecular imaging of disease with targeted contrast ultrasound imaging. Medical Solutions. 2005. 57–63.16. Villanueva FS, Jankowski RJ, Klibanov S, Pina ML, Alber SM, Watkins SC, Brandenburger GH, Wagner WR. Microbubbles targeted to intercellular adhesion molecule-1 bind to activated coronary artery endothelial cells. Circulation. 1998. 98:1–5.
Article17. Wei K, Skyba DM, Firschke C, Jayaweera AR, Lindner JR, Kaul S. Interactions between microbubbles and ultrasound: in vitro and in vivo observations. J Am Coll Cardiol. 1997. 29:1081–1088.
Article18. Chomas JE, Dayton P, Allen J, Morgan K, Ferrara KW. Mechanisms of contrast agent destruction. IEEE Trans Ultrason Ferroelectr Freq Control. 2001. 48:232–248.
Article19. de Jong N, Cornet R, Lancee CT. Higher harmonics of vibrating gas-filled microspheres. Part two: measurements. Ultrasonics. 1994. 32:455–459.
Article20. Hoff L, Sontum PC, Hoff B. Acoustic properties of shell-encapsulated, gas-filled ultrasound contrast agents. Proc IEEE Ultrason Symp. 1996. 2:1441–1444.
Article21. Lindner JR, Dayton PA, Coggins MP, Ley K, Song J, Ferrara K, Kaul S. Noninvasive imaging of inflammation by ultrasound detection of phagocytosed microbubbles. Circulation. 2000. 102:531–538.
Article22. Dayton PA, Chomas JE, Lum AF, Allen JS, Lindner JR, Simon SI, Ferrara KW. Optical and acoustical dynamics of microbubble contrast agents inside neutrophils. Biophys J. 2001. 80:1547–1556.
Article23. Kaufmann BA, Carr CL, Belcik T, Xie A, Kron B, Yue Q, Lindner JR. Effect of acoustic power on in vivo molecular imaging with targeted microbubbles: implications for low-mechanical index real-time imaging. J Am Soc Echocardiogr. 2010. 23:79–85.
Article24. Leong-Poi H, Coggins MP, Sklenar J, Jayaweera AR, Wang XQ, Kaul S. Role of collateral blood flow in the apparent disparity between the extent of abnormal wall thickening and perfusion defect size during acute myocardial infarction and demand ischemia. J Am Coll Cardiol. 2005. 45:565–572.
Article25. Kang DH, Kang SJ, Song JM, Choi KJ, Hong MK, Song JK, Park SW, Park SJ. Efficacy of myocardial contrast echocardiography in the diagnosis and risk stratification of acute coronary syndrome. Am J Cardiol. 2005. 96:1498–1502.
Article26. Ley K, Bullard DC, Arbonés ML, Bosse R, Vestweber D, Tedder TF, Beaudet AL. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med. 1995. 181:669–675.
Article27. Kaufmann BA, Lewis C, Xie A, Mirza-Mohd A, Lindner JR. Detection of recent myocardial ischaemia by molecular imaging of P-selectin with targeted contrast echocardiography. Eur Heart J. 2007. 28:2011–2017.
Article28. Christiansen JP, Leong-Poi H, Klibanov AL, Kaul S, Lindner JR. Noninvasive imaging of myocardial reperfusion injury using leukocyte-targeted contrast echocardiography. Circulation. 2002. 105:1764–1767.
Article29. Yan Y, Liao Y, Yang L, Wu J, Du J, Xuan W, Ji L, Huang Q, Liu Y, Bin J. Late-phase detection of recent myocardial ischaemia using ultrasound molecular imaging targeted to intercellular adhesion molecule-1. Cardiovasc Res. 2011. 89:175–183.
Article30. Weller GE, Villanueva FS, Klibanov AL, Wagner WR. Modulating targeted adhesion of an ultrasound contrast agent to dysfunctional endothelium. Ann Biomed Eng. 2002. 30:1012–1019.
Article31. Libby P. Inflammation in atherosclerosis. Nature. 2002. 420:868–874.
Article32. Kaufmann BA, Sanders JM, Davis C, Xie A, Aldred P, Sarembock IJ, Lindner JR. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007. 116:276–284.
Article33. Lyshchik A, Fleischer AC, Huamani J, Hallahan DE, Brissova M, Gore JC. Molecular imaging of vascular endothelial growth factor receptor 2 expression using targeted contrast-enhanced high-frequency ultrasonography. J Ultrasound Med. 2007. 26:1575–1586.
Article34. Palmowski M, Huppert J, Ladewig G, Hauff P, Reinhardt M, Mueller MM, Woenne EC, Jenne JW, Maurer M, Kauffmann GW, Semmler W, Kiessling F. Molecular profiling of angiogenesis with targeted ultrasound imaging: early assessment of antiangiogenic therapy effects. Mol Cancer Ther. 2008. 7:101–109.
Article35. Weller GE, Wong MK, Modzelewski RA, Lu E, Klibanov AL, Wagner WR, Villanueva FS. Ultrasonic imaging of tumor angiogenesis using contrast microbubbles targeted via the tumor-binding peptide arginine-arginine-leucine. Cancer Res. 2005. 65:533–539.36. Leong-Poi H, Christiansen J, Klibanov AL, Kaul S, Lindner JR. Noninvasive assessment of angiogenesis by ultrasound and microbubbles targeted to alpha(v)-integrins. Circulation. 2003. 107:455–460.
Article37. Stieger SM, Dayton PA, Borden MA, Caskey CF, Griffey SM, Wisner ER, Ferrara KW. Imaging of angiogenesis using Cadence contrast pulse sequencing and targeted contrast agents. Contrast Media Mol Imaging. 2008. 3:9–18.
Article38. Leong-Poi H, Christiansen J, Heppner P, Lewis CW, Klibanov AL, Kaul S, Lindner JR. Assessment of endogenous and therapeutic arteriogenesis by contrast ultrasound molecular imaging of integrin expression. Circulation. 2005. 111:3248–3254.
Article39. Behm CZ, Kaufmann BA, Carr C, Lankford M, Sanders JM, Rose CE, Kaul S, Lindner JR. Molecular imaging of endothelial vascular cell adhesion molecule-1 expression and inflammatory cell recruitment during vasculogenesis and ischemia-mediated arteriogenesis. Circulation. 2008. 117:2902–2911.
Article40. Christiansen JP, French BA, Klibanov AL, Kaul S, Lindner JR. Targeted tissue transfection with ultrasound destruction of plasmid-bearing cationic microbubbles. Ultrasound Med Biol. 2003. 29:1759–1767.
Article41. Taniyama Y, Tachibana K, Hiraoka K, Namba T, Yamasaki K, Hashiya N, Aoki M, Ogihara T, Yasufumi K, Morishita R. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation. 2002. 105:1233–1239.
Article42. Porter TR, Hiser WL, Kricsfeld D, Deligonul U, Xie F, Iversen P, Radio S. Inhibition of carotid artery neointimal formation with intravenous microbubbles. Ultrasound Med Biol. 2001. 27:259–265.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Primer on the Methods and Applications for Contrast Echocardiography in Clinical Imaging
- Clinical use of contrast-enhanced ultrasound beyond the liver: a focus on renal, splenic, and pancreatic applications
- Present status and perspectives of endosonography 2017 in gastroenterology
- Ultrasound Contrast Agent
- Microbubbles used for contrast enhanced ultrasound and theragnosis: a review of principles to applications