Diabetes Metab J.
2011 Oct;35(5):458-465. 10.4093/dmj.2011.35.5.458.
Fuel-Stimulated Insulin Secretion Depends upon Mitochondria Activation and the Integration of Mitochondrial and Cytosolic Substrate Cycles
- Affiliations
-
- 1Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, USA. gary.cline@yale.edu
- KMID: 1857500
- DOI: http://doi.org/10.4093/dmj.2011.35.5.458
Abstract
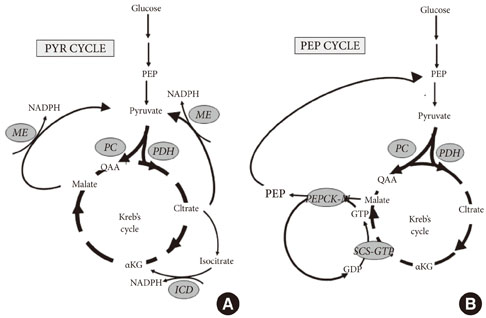
- The pancreatic islet beta-cell is uniquely specialized to couple its metabolism and rates of insulin secretion with the levels of circulating nutrient fuels, with the mitochondrial playing a central regulatory role in this process. In the beta-cell, mitochondrial activation generates an integrated signal reflecting rates of oxidativephosphorylation, Kreb's cycle flux, and anaplerosis that ultimately determines the rate of insulin exocytosis. Mitochondrial activation can be regulated by proton leak and mediated by UCP2, and by alkalinization to utilize the pH gradient to drive substrate and ion transport. Converging lines of evidence support the hypothesis that substrate cycles driven by rates of Kreb's cycle flux and by anaplerosis play an integral role in coupling responsive changes in mitochondrial metabolism with insulin secretion. The components and mechanisms that account for the integrated signal of ATP production, substrate cycling, the regulation of cellular redox state, and the production of other secondary signaling intermediates are operative in both rodent and human islet beta-cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Henquin JC, Ravier MA, Nenquin M, Jonas JC, Gilon P. Hierarchy of the beta-cell signals controlling insulin secretion. Eur J Clin Invest. 2003. 33:742–750.2. Antinozzi PA, Ishihara H, Newgard CB, Wollheim CB. Mitochondrial metabolism sets the maximal limit of fuel-stimulated insulin secretion in a model pancreatic beta cell: a survey of four fuel secretagogues. J Biol Chem. 2002. 277:11746–11755.3. Aguilar-Bryan L, Bryan J. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev. 1999. 20:101–135.4. MacDonald MJ. Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. Further implication of cytosolic NADPH in insulin secretion. J Biol Chem. 1995. 270:20051–20058.5. Farfari S, Schulz V, Corkey B, Prentki M. Glucose-regulated anaplerosis and cataplerosis in pancreatic beta-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes. 2000. 49:718–726.6. Lu D, Mulder H, Zhao P, Burgess SC, Jensen MV, Kamzolova S, Newgard CB, Sherry AD. 13C NMR isotopomer analysis reveals a connection between pyruvate cycling and glucose-stimulated insulin secretion (GSIS). Proc Natl Acad Sci USA. 2002. 99:2708–2713.7. Cline GW, Lepine RL, Papas KK, Kibbey RG, Shulman GI. 13C NMR isotopomer analysis of anaplerotic pathways in INS-1 cells. J Biol Chem. 2004. 279:44370–44375.8. Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001. 105:745–755.9. Krauss S, Zhang CY, Scorrano L, Dalgaard LT, St-Pierre J, Grey ST, Lowell BB. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J Clin Invest. 2003. 112:1831–1842.10. Pi J, Bai Y, Daniel KW, Liu D, Lyght O, Edelstein D, Brownlee M, Corkey BE, Collins S. Persistent oxidative stress due to absence of uncoupling protein 2 associated with impaired pancreatic beta-cell function. Endocrinology. 2009. 150:3040–3048.11. Pongratz RL, Kibbey RG, Kirkpatrick CL, Zhao X, Pontoglio M, Yaniv M, Wollheim CB, Shulman GI, Cline GW. Mitochondrial dysfunction contributes to impaired insulin secretion in INS-1 cells with dominant-negative mutations of HNF-1alpha and in HNF-1alpha-deficient islets. J Biol Chem. 2009. 284:16808–16821.12. Kjorholt C, Akerfeldt MC, Biden TJ, Laybutt DR. Chronic hyperglycemia, independent of plasma lipid levels, is sufficient for the loss of beta-cell differentiation and secretory function in the db/db mouse model of diabetes. Diabetes. 2005. 54:2755–2763.13. Palmieri F. The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflugers Arch. 2004. 447:689–709.14. Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009. 326:144–147.15. Wiederkehr A, Park KS, Dupont O, Demaurex N, Pozzan T, Cline GW, Wollheim CB. Matrix alkalinization: a novel mitochondrial signal for sustained pancreatic beta-cell activation. EMBO J. 2009. 28:417–428.16. Maechler P, Wollheim CB. Mitochondrial function in normal and diabetic beta-cells. Nature. 2001. 414:807–812.17. Simpson DP, Hager SR. Bicarbonate-carbon dioxide buffer system: a determinant of the mitochondrial pH gradient. Am J Physiol. 1984. 247(3 Pt 2):F440–F446.18. Wiederkehr A, Wollheim CB. Impact of mitochondrial calcium on the coupling of metabolism to insulin secretion in the pancreatic beta-cell. Cell Calcium. 2008. 44:64–76.19. Pongratz RL, Kibbey RG, Shulman GI, Cline GW. Cytosolic and mitochondrial malic enzyme isoforms differentially control insulin secretion. J Biol Chem. 2007. 282:200–207.20. Liu YQ, Jetton TL, Leahy JL. Beta-Cell adaptation to insulin resistance: increased pyruvate carboxylase and malate-pyruvate shuttle activity in islets of nondiabetic Zucker fatty rats. J Biol Chem. 2002. 277:39163–39168.21. Ishihara H, Wang H, Drewes LR, Wollheim CB. Overexpression of monocarboxylate transporter and lactate dehydrogenase alters insulin secretory responses to pyruvate and lactate in beta cells. J Clin Invest. 1999. 104:1621–1629.22. Ashcroft SJ, Christie MR. Effects of glucose on the cytosolic ration of reduced/oxidized nicotinamide-adenine dinucleotide phosphate in rat islets of Langerhans. Biochem J. 1979. 184:697–700.23. Ivarsson R, Quintens R, Dejonghe S, Tsukamoto K, in't Veld P, Renstrom E, Schuit FC. Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes. 2005. 54:2132–2142.24. Joseph JW, Jensen MV, Ilkayeva O, Palmieri F, Alarcon C, Rhodes CJ, Newgard CB. The mitochondrial citrate/isocitrate carrier plays a regulatory role in glucose-stimulated insulin secretion. J Biol Chem. 2006. 281:35624–35632.25. Ronnebaum SM, Ilkayeva O, Burgess SC, Joseph JW, Lu D, Stevens RD, Becker TC, Sherry AD, Newgard CB, Jensen MV. A pyruvate cycling pathway involving cytosolic NADP-dependent isocitrate dehydrogenase regulates glucose-stimulated insulin secretion. J Biol Chem. 2006. 281:30593–30602.26. Ronnebaum SM, Jensen MV, Hohmeier HE, Burgess SC, Zhou YP, Qian S, MacNeil D, Howard A, Thornberry N, Ilkayeva O, Lu D, Sherry AD, Newgard CB. Silencing of cytosolic or mitochondrial isoforms of malic enzyme has no effect on glucosestimulated insulin secretion from rodent islets. J Biol Chem. 2008. 283:28909–28917.27. Fell D. Understanding the control of metabolism. 1997. London: Portland Press Ltd.;101–133. Chapter 5, Metabolic control analysis.28. Brown LJ, Longacre MJ, Hasan NM, Kendrick MA, Stoker SW, Macdonald MJ. Chronic reduction of the cytosolic or mitochondrial NAD(P)-malic enzyme does not affect insulin secretion in a rat insulinoma cell line. J Biol Chem. 2009. 284:35359–35367.29. MacDonald PE, Joseph JW, Rorsman P. Glucose-sensing mechanisms in pancreatic beta-cells. Philos Trans R Soc Lond B Biol Sci. 2005. 360:2211–2225.30. Mandella RD, Sauer LA. The mitochondrial malic enzymes. I. Submitochondrial localization and purification and properties of the NAD(P)+-dependent enzyme from adrenal cortex. J Biol Chem. 1975. 250:5877–5884.31. Teller JK, Fahien LA, Davis JW. Kinetics and regulation of hepatoma mitochondrial NAD(P) malic enzyme. J Biol Chem. 1992. 267:10423–10432.32. Kibbey RG, Pongratz RL, Romanelli AJ, Wollheim CB, Cline GW, Shulman GI. Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab. 2007. 5:253–264.33. Stark R, Pasquel F, Turcu A, Pongratz RL, Roden M, Cline GW, Shulman GI, Kibbey RG. Phosphoenolpyruvate cycling via mitochondrial phosphoenolpyruvate carboxykinase links anaplerosis and mitochondrial GTP with insulin secretion. J Biol Chem. 2009. 284:26578–26590.34. Pongratz RL, Papas KK, Kibbey RG, Cline GW. Role for anaplerosis and pyruvate cycling in fuel-stimulated insulin secretion from human islets. Paper presented at: Keystone Symposia: Islet and Beta Cell Biology. 2008 Apr 6-11; Snowbird, UT, USA.35. MacDonald MJ, Longacre MJ, Stoker SW, Kendrick M, Thonpho A, Brown LJ, Hasan NM, Jitrapakdee S, Fukao T, Hanson MS, Fernandez LA, Odorico J. Differences between human and rodent pancreatic islets: low pyruvate carboxylase, atp citrate lyase, and pyruvate carboxylation and high glucose-stimulated acetoacetate in human pancreatic islets. J Biol Chem. 2011. 286:18383–18396.36. Papas KK, Jarema MA. Glucose-stimulated insulin secretion is not obligatorily linked to an increase in O2 consumption in betaHC9 cells. Am J Physiol. 1998. 275(6 Pt 1):E1100–E1106.37. Cline GW, Pongratz RL, Zhao X, Papas KK. Rates of insulin secretion in INS-1 cells are enhanced by coupling to anaplerosis and Kreb's cycle flux independent of ATP synthesis. Biochem Biophys Res Commun. Epub 2011 Oct 7. DOI:10.1016/j.bbrc.2011.09.153.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glucagon-Like Peptide-1 Increases Mitochondrial Biogenesis and Function in INS-1 Rat Insulinoma Cells
- Defective Mitochondrial Function and Motility Due to Mitofusin 1 Overexpression in Insulin Secreting Cells
- Control of Mitochondrial Dynamics by Fas-induced Caspase-8 Activation in Hippocampal Neurons
- Effects of Antioxidants on Ethidium Bromide-induced Inhibition of Insulin Secretion in Rat Pancreatic Islets
- miR-24-mediated knockdown of H2AX damages mitochondria and the insulin signaling pathway