Korean J Physiol Pharmacol.
2012 Feb;16(1):71-77. 10.4196/kjpp.2012.16.1.71.
Defective Mitochondrial Function and Motility Due to Mitofusin 1 Overexpression in Insulin Secreting Cells
- Affiliations
-
- 1Department of Physiology, Institute of Lifestyle Medicine, Yonsei University Wonju College of Medicine, Wonju 220-701, Korea. qsang@yonsei.ac.kr
- 2Nestle Institute of Health Sciences, 1015 Lausanne, Switzerland.
- 3Department of Cell Physiology and Metabolism, University of Geneva, 1211 Geneva, Switzerland.
- KMID: 2285443
- DOI: http://doi.org/10.4196/kjpp.2012.16.1.71
Abstract
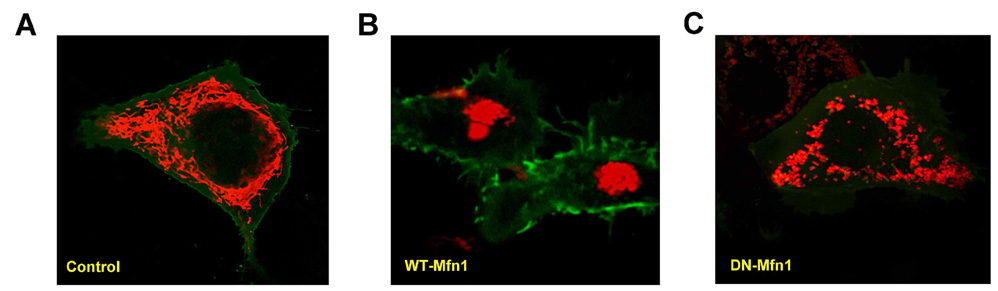
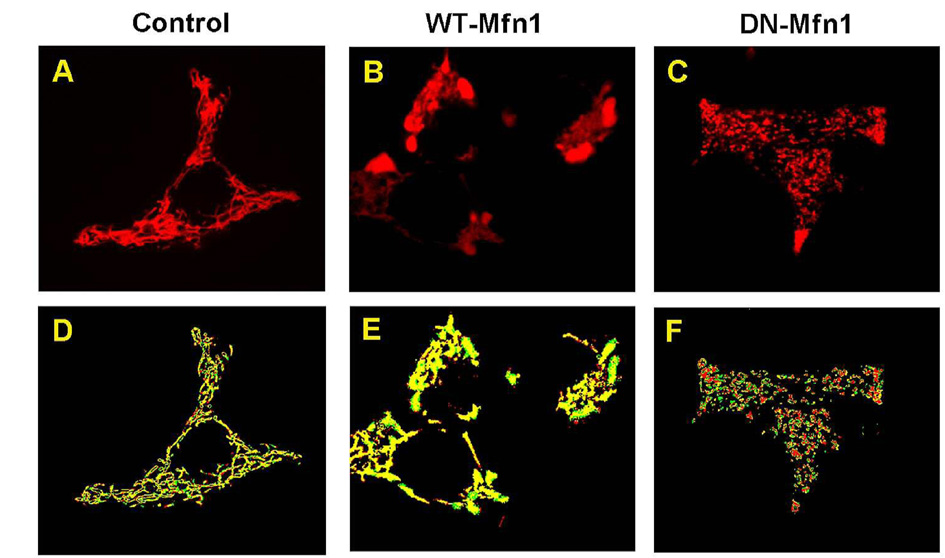
- Mitochondrial dynamics and distribution is critical for their role in bioenergetics and cell survival. We investigated the consequence of altered fission/fusion on mitochondrial function and motility in INS-1E rat clonal beta-cells. Adenoviruses were used to induce doxycycline-dependent expression of wild type (WT-Mfn1) or a dominant negative mitofusin 1 mutant (DN-Mfn1). Mitochondrial morphology and motility were analyzed by monitoring mitochondrially-targeted red fluorescent protein. Adenovirus-driven overexpression of WT-Mfn1 elicited severe aggregation of mitochondria, preventing them from reaching peripheral near plasma membrane areas of the cell. Overexpression of DN-Mfn1 resulted in fragmented mitochondria with widespread cytosolic distribution. WT-Mfn1 overexpression impaired mitochondrial function as glucose- and oligomycin-induced mitochondrial hyperpolarization were markedly reduced. Viability of the INS-1E cells, however, was not affected. Mitochondrial motility was significantly reduced in WT-Mfn1 overexpressing cells. Conversely, fragmented mitochondria in DN-Mfn1 overexpressing cells showed more vigorous movement than mitochondria in control cells. Movement of these mitochondria was also less microtubule-dependent. These results suggest that Mfn1-induced hyperfusion leads to mitochondrial dysfunction and hypomotility, which may explain impaired metabolism-secretion coupling in insulin-releasing cells overexpressing Mfn1.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Comparison of Ectopic Gene Expression Methods in Rat Neural Stem Cells
Woosuk Kim, Ji Hyeon Kim, Sun-Young Kong, Min-Hye Park, Uy Dong Sohn, Hyun-Jung Kim
Korean J Physiol Pharmacol. 2013;17(1):23-30. doi: 10.4196/kjpp.2013.17.1.23.
Reference
-
1. Maechler P, Wollheim CB. Mitochondrial function in normal and diabetic beta-cells. Nature. 2001. 414:807–812.2. Wiederkehr A, Wollheim CB. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology. 2006. 147:2643–2649.3. Wiederkehr A, Wollheim CB. Mitochondrial signals drive insulin secretion in the pancreatic β-cell. Mol Cell Endocrinol. 2011. [Epub ahead of print].4. Maassen JA, Janssen GM, 't Hart LM. Molecular mechanisms of mitochondrial diabetes (MIDD). Ann Med. 2005. 37:213–221.5. Del Guerra S, Lupi R, Marselli L, Masini M, Bugliani M, Sbrana S, Torri S, Pollera M, Boggi U, Mosca F, DelPrato S, Marchetti P. Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes. 2005. 54:727–735.6. Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007. 8:870–879.7. Yoon Y, Pitts KR, McNiven MA. Mammalian dynamin-like protein DLP1 tubulates membranes. Mol Biol Cell. 2001. 12:2894–2905.8. James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003. 278:36373–36379.9. Otera H, Mihara K. Molecular mechanisms and physiologic functions of mitochondrial dynamics. J Biochem. 2011. 149:241–251.10. Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, Youle RJ, Mihara K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol. 2010. 191:1141–1158.11. Lee Y, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004. 15:5001–5011.12. Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008. 27:433–446.13. Santel A, Frank S, Gaume B, Herrler M, Youle RJ, Fuller MT. Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. J Cell Sci. 2003. 116:2763–2774.14. Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003. 160:189–200.15. Züchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, Zappia M, Nelis E, Patitucci A, Senderek J, Parman Y, Evgrafov O, Jonghe PD, Takahashi Y, Tsuji S, Pericak-Vance MA, Quattrone A, Battaloglu E, Polyakov AV, Timmerman V, Schröder JM, Vance JM. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004. 36:449–451.16. Reynolds IJ, Rintoul GL. Mitochondrial stop and go: signals that regulate organelle movement. Sci STKE. 2004. 2004:PE46.17. Boldogh IR, Pon LA. Mitochondria on the move. Trends Cell Biol. 2007. 17:502–510.18. Frederick RL, Shaw JM. Moving mitochondria: establishing distribution of an essential organelle. Traffic. 2007. 8:1668–1675.19. Russo GJ, Louie K, Wellington A, Macleod GT, Hu F, Panchumarthi S, Zinsmaier KE. Drosophila Miro is required for both anterograde and retrograde axonal mitochondrial transport. J Neurosci. 2009. 29:5443–5455.20. Yi M, Weaver D, Hajnóczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol. 2004. 167:661–672.21. Wollheim CB. Beta-cell mitochondria in the regulation of insulin secretion: a new culprit in type II diabetes. Diabetologia. 2000. 43:265–277.22. Park KS, Wiederkehr A, Kirkpatrick C, Mattenberger Y, Martinou JC, Marchetti P, Demaurex N, Wollheim CB. Selective actions of mitochondrial fission/fusion genes on metabolismsecretion coupling in insulin-releasing cells. J Biol Chem. 2008. 283:33347–33356.23. Brun T, Franklin I, St-Onge L, Biason-Lauber A, Schoenle EJ, Wollheim CB, Gauthier BR. The diabetes-linked transcription factor PAX4 promotes {beta}-cell proliferation and survival in rat and human islets. J Cell Biol. 2004. 167:1123–1135.24. Irwin WA, Bergamin N, Sabatelli P, Reggiani C, Megighian A, Merlini L, Braghetta P, Columbaro M, Volpin D, Bressan GM, Bernardi P, Bonaldo P. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat Genet. 2003. 35:367–371.25. Brough D, Schell MJ, Irvine RF. Agonist-induced regulation of mitochondrial and endoplasmic reticulum motility. Biochem J. 2005. 392:291–297.26. Youm JB, Choi SW, Jang CH, Kim HK, Leem CH, Kim N, Han J. A computational model of cytosolic and mitochondrial [ca] in paced rat ventricular myocytes. Korean J Physiol Pharmacol. 2011. 15:217–239.27. Chen H, Chan DC. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum Mol Genet. 2009. 18:R169–R176.28. Pich S, Bach D, Briones P, Liesa M, Camps M, Testar X, Palacín M, Zorzano A. The Charcot-Marie-Tooth type 2A gene product, Mfn2, up-regulates fuel oxidation through expression of OXPHOS system. Hum Mol Genet. 2005. 14:1405–1415.29. de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008. 456:605–610.30. Singaravelu K, Nelson C, Bakowski D, de Brito OM, Ng SW, Di Capite J, Powell T, Scorrano L, Parekh AB. Mitofusin 2 regulates STIM1 migration from the Ca2+ store to the plasma membrane in cells with depolarized mitochondria. J Biol Chem. 2011. 286:12189–12201.31. Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004. 117:6535–6546.32. Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009. 11:958–966.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Deficiency of Sphingosine-1-Phosphate Reduces the Expression of Prohibitin and Causes β-Cell Impairment via Mitochondrial Dysregulation
- Autophagy in Diabetes
- Overexpression of SIRT3 Suppresses Oxidative Stress-induced Neurotoxicity and Mitochondrial Dysfunction in Dopaminergic Neuronal Cells
- Glucagon-Like Peptide-1 Increases Mitochondrial Biogenesis and Function in INS-1 Rat Insulinoma Cells
- Exercise and Mitochondrial Remodeling in Skeletal Muscle in Type 2 Diabetes