Korean J Urol.
2013 Aug;54(8):510-515. 10.4111/kju.2013.54.8.510.
Do Positive Surgical Margins Predict Biochemical Recurrence in All Patients Without Adjuvant Therapy After Radical Prostatectomy?
- Affiliations
-
- 1Department of Urology, Veterans Health Service Medical Center, Seoul, Korea. urodoct@hotmail.com
- 2Department of Urology, Asan Health Center, Asan, Korea.
- KMID: 1840462
- DOI: http://doi.org/10.4111/kju.2013.54.8.510
Abstract
- PURPOSE
The objective was to study whether positive surgical margins (PSMs) predict biochemical recurrence (BCR) in all patients without adjuvant therapy after radical prostatectomy (RP).
MATERIALS AND METHODS
We retrospectively reviewed the medical records of patients who underwent RP for prostate cancer at Veterans Health Service Medical Center from 2005 to 2011. BCR was defined by a prostate-specific antigen (PSA) value > or =0.2 ng/mL. The clinicopathological factors of the PSM group were compared with those of the negative surgical margin (NSM) group, and the predictive impact of a PSM for BCR-free survival were evaluated. In addition, we analyzed the prognostic difference for BCR-free survival between solitary and multiple PSMs.
RESULTS
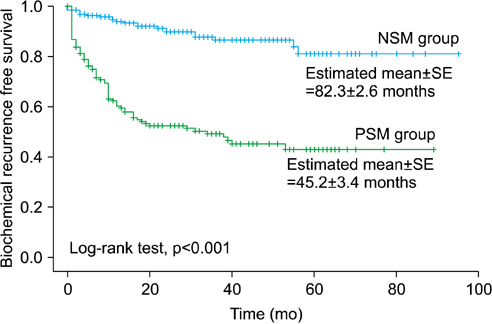
A PSM was noted in 167 patients (45.5%). BCR was reported in 101 men in total (27.5%). The BCR-free survival rate of the PSM group was lower than that of the NSM group (p<0.001). In a multivariate analysis for the total patients, PSM was significantly associated with BCR-free survival (p<0.001). After stratification by pathological T stage, Gleason score (GS), and preoperative PSA value, PSM was significantly predictive for BCR-free survival in men with pT2 and/or GS < or =6 or 7 and/or a PSA value <10 or 10-20 ng/mL (all p<0.05). Multiple PSMs were more predictive of BCR-free survival than was a solitary PSM (p=0.001).
CONCLUSIONS
A PSM is a significant predictor of postoperative BCR in patients with pT2 and/or GS < or =7 and/or preoperative PSA <20 ng/mL. Multiple PSMs are considered a stronger prognostic factor for prediction of BCR than is a solitary PSM.
Keyword
MeSH Terms
Figure
Reference
-
1. Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Seo HG, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012. 44:11–24.2. Bastian PJ, Boorjian SA, Bossi A, Briganti A, Heidenreich A, Freedland SJ, et al. High-risk prostate cancer: from definition to contemporary management. Eur Urol. 2012. 61:1096–1106.3. Grubb RL, Kibel AS. High-risk localized prostate cancer: role of radical prostatectomy. Curr Opin Urol. 2010. 20:204–210.4. Sundi D, Jeong BC, Lee SB, Han M. Optimizing the management of high-risk, localized prostate cancer. Korean J Urol. 2012. 53:815–820.5. Blute ML, Bergstralh EJ, Iocca A, Scherer B, Zincke H. Use of Gleason score, prostate specific antigen, seminal vesicle and margin status to predict biochemical failure after radical prostatectomy. J Urol. 2001. 165:119–125.6. Ro YK, Lee S, Jeong CW, Hong SK, Byun SS, Lee SE. Biochemical recurrence in Gleason score 7 prostate cancer in korean men: significance of the primary Gleason grade. Korean J Urol. 2012. 53:826–829.7. Epstein JI, Partin AW, Potter SR, Walsh PC. Adenocarcinoma of the prostate invading the seminal vesicle: prognostic stratification based on pathologic parameters. Urology. 2000. 56:283–288.8. Nelson BA, Shappell SB, Chang SS, Wells N, Farnham SB, Smith JA Jr, et al. Tumour volume is an independent predictor of prostate-specific antigen recurrence in patients undergoing radical prostatectomy for clinically localized prostate cancer. BJU Int. 2006. 97:1169–1172.9. Uhlman MA, Sun L, Stackhouse DA, Caire AA, Polascik TJ, Robertson CN, et al. Tumor volume, tumor percentage involvement, or prostate volume: which is predictive of prostate-specific antigen recurrence? Urology. 2010. 75:460–466.10. Corcoran NM, Hovens CM, Metcalfe C, Hong MK, Pedersen J, Casey RG, et al. Positive surgical margins are a risk factor for significant biochemical recurrence only in intermediate-risk disease. BJU Int. 2012. 110:821–827.11. Ploussard G, Agamy MA, Alenda O, Allory Y, Mouracade P, Vordos D, et al. Impact of positive surgical margins on prostate-specific antigen failure after radical prostatectomy in adjuvant treatment-naïve patients. BJU Int. 2011. 107:1748–1754.12. Chalfin HJ, Dinizo M, Trock BJ, Feng Z, Partin AW, Walsh PC, et al. Impact of surgical margin status on prostate-cancer-specific mortality. BJU Int. 2012. 110:1684–1689.13. So BK, Choi JD, Lee SY, Kim HS, Park SY, Seo SI. Experience of 100 laparoscopic radical prostatectomies performed by a single surgeon: an analysis of surgical and functional outcomes. Korean J Urol. 2011. 52:517–523.14. Oh JJ, Hong SK, Byun SS, Choe G, Lee SE. Prognostic significance of positive surgical margins after radical prostatectomy among pT2 and pT3a prostate cancer. Urol Oncol. 2013. 31:595–600.15. Mauermann J, Fradet V, Lacombe L, Dujardin T, Tiguert R, Tetu B, et al. The Impact of solitary and multiple positive surgical margins on hard clinical end points in 1712 adjuvant treatment-naive pT2-4 N0 radical prostatectomy patients. Eur Urol. 2013. 64:19–25.16. Wieder JA, Soloway MS. Incidence, etiology, location, prevention and treatment of positive surgical margins after radical prostatectomy for prostate cancer. J Urol. 1998. 160:299–315.17. Tan PH, Cheng L, Srigley JR, Griffiths D, Humphrey PA, van der Kwast TH, et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 5: surgical margins. Mod Pathol. 2011. 24:48–57.18. Epstein JI. Incidence and significance of positive margins in radical prostatectomy specimens. Urol Clin North Am. 1996. 23:651–663.19. Eminaga O, Hinkelammert R, Titze U, Abbas M, Eltze E, Bettendorf O, et al. The presence of positive surgical margins in patients with organ-confined prostate cancer results in biochemical recurrence at a similar rate to that in patients with extracapsular extension and PSA≤10 ng/ml. Urol Oncol. 2013. 02. 19. [Epub]. http://dx.doi.org/10.1016/j.urolonc.2012.11.021.20. Stamey TA, McNeal JE, Yemoto CM, Sigal BM, Johnstone IM. Biological determinants of cancer progression in men with prostate cancer. JAMA. 1999. 281:1395–1400.21. Vis AN, Schroder FH, van der Kwast TH. The actual value of the surgical margin status as a predictor of disease progression in men with early prostate cancer. Eur Urol. 2006. 50:258–265.22. Stephenson AJ, Wood DP, Kattan MW, Klein EA, Scardino PT, Eastham JA, et al. Location, extent and number of positive surgical margins do not improve accuracy of predicting prostate cancer recurrence after radical prostatectomy. J Urol. 2009. 182:1357–1363.23. Marks RA, Koch MO, Lopez-Beltran A, Montironi R, Juliar BE, Cheng L. The relationship between the extent of surgical margin positivity and prostate specific antigen recurrence in radical prostatectomy specimens. Hum Pathol. 2007. 38:1207–1211.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Impact of Positive Surgical Margins on Biochemical Recurrence after Radical Retropubic Prostatectomy
- Influences of Neoadjuvant Androgen Ablation before Radical Prostatectomy on Positive Surgical Margin and Biochemical Recurrence Rate
- The Inpact of Positive Surgical Margins and Their Preoperative Predicting Factors on Biochemical Failure after Radical Retropubic Prostatectomy
- The Effect of Tumor-Prostate Ratio on Biochemical Recurrence after Radical Prostatectomy
- Cribriform Pattern at the Surgical Margin is Highly Predictive of Biochemical Recurrence in Patients Undergoing Radical Prostatectomy