Korean Circ J.
2010 Oct;40(10):479-488. 10.4070/kcj.2010.40.10.479.
Pericardial Approach for Cardiac Therapies: Old Practice With New Ideas
- Affiliations
-
- 1Utah Valley Regional Medical Center, Provo, UT, USA. chunhwangmd@aol.com
- 2Krannert Institute of Cardiology, Indiana University School of Medicine, Indianapolis, IN, USA.
- KMID: 1826135
- DOI: http://doi.org/10.4070/kcj.2010.40.10.479
Abstract
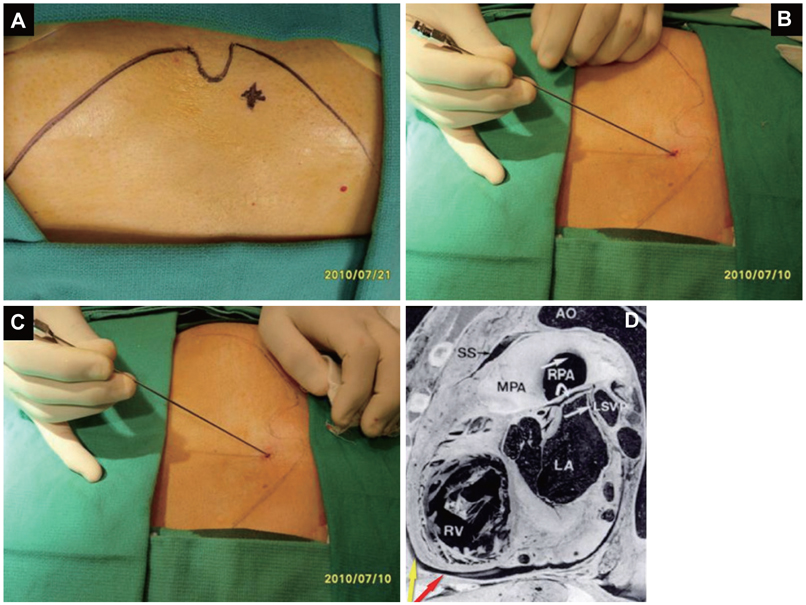
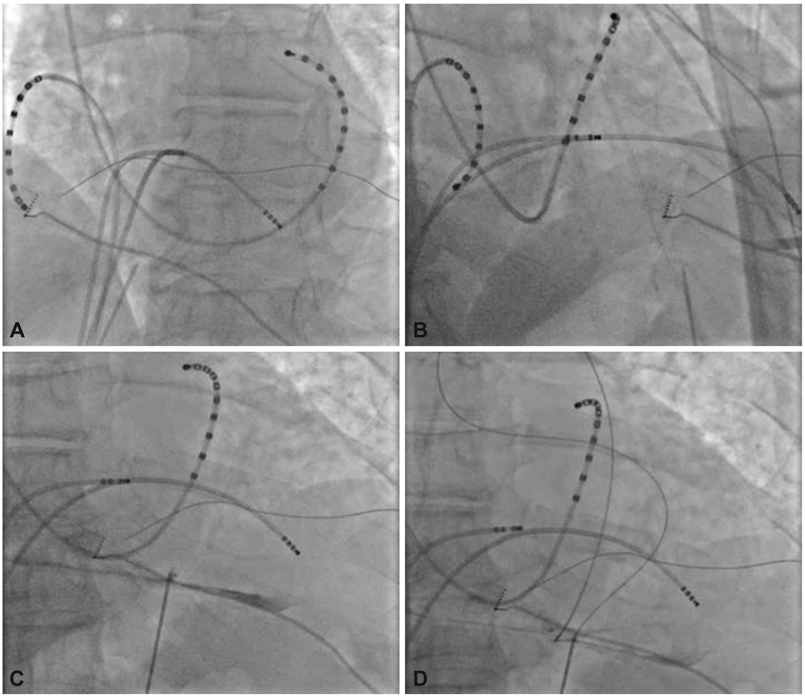
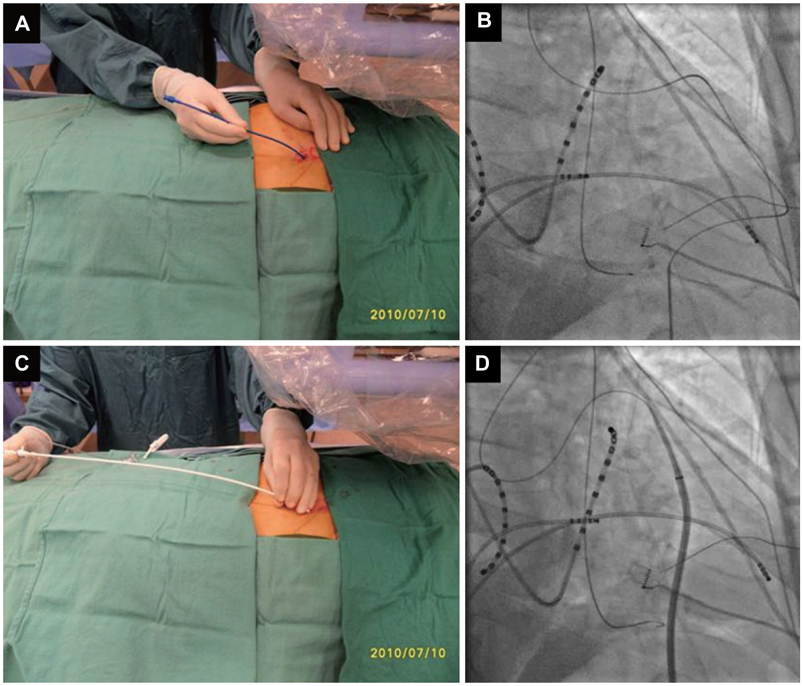
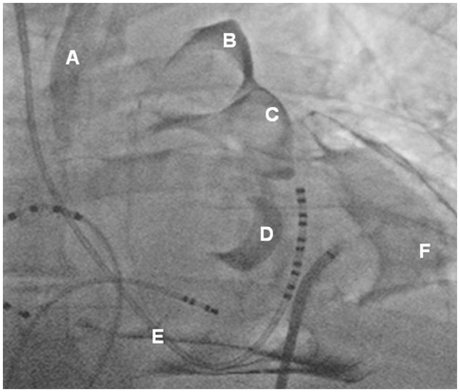
- Treatment of cardiac disease via the epicardium fell under the domain of cardiac surgery due to the need for an open thoracotomy. Since an open thoracotomy is invasive in nature and has the potential for complications, a minimally invasive and percutaneous approach would be more attractive for suitable patients. The recent success of epicardial ablation of refractory arrhythmia via the percutaneous pericardial approach has increased the potential for delivery of epicardial therapies. Epicardial ablation has increased the success and safety since anti-coagulation and transseptal catheterization for left atrial arrhythmias is not required. The pericardial space has also been used to deliver therapy for several cardiac diseases. There are reports on successful delivery of drugs and their efficacy. Even though there was a wide range of efficacies reported in those studies, the reported complication rates are strikingly low, which suggests that direct delivery of drugs to the epicardium via the pericardial space is safe. Furthermore, recent animal studies have supported the feasibility of epicardial delivery of biological agents, including genes, cells, and even genetically engineered tissue for therapeutic purposes. In conclusion, percutaneous pericardial cannulation of closed pericardial space can play a significant role in providing non-surgical therapy for cardiovascular diseases. However, it requires skills and operator experiences. Therefore, there is need to further develop new tools, safer techniques, and effective procedure environment before generalizing this procedure.
MeSH Terms
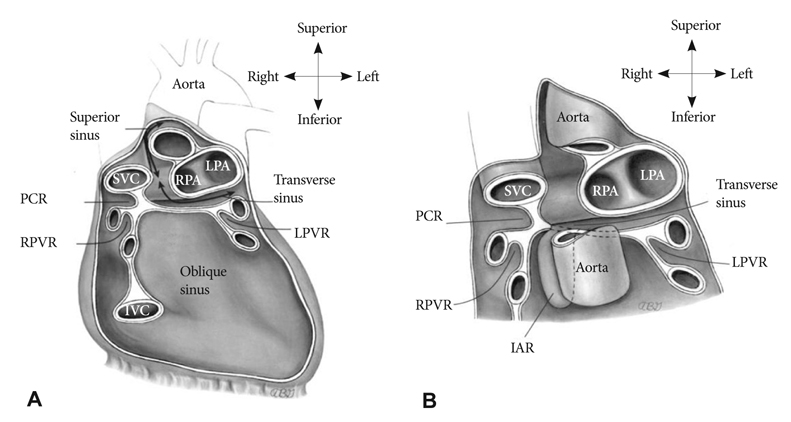
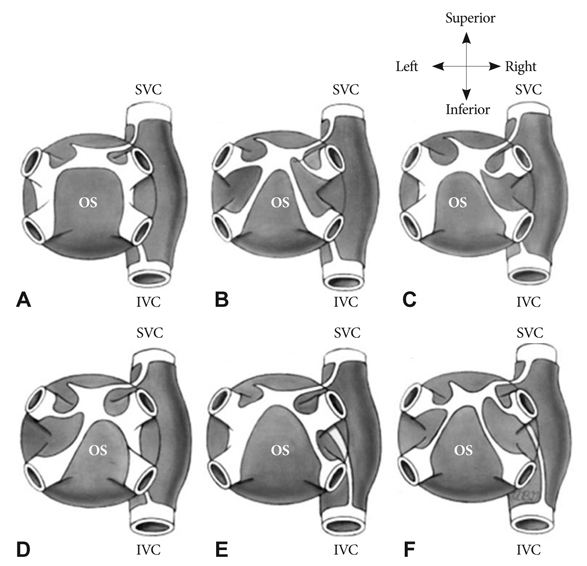
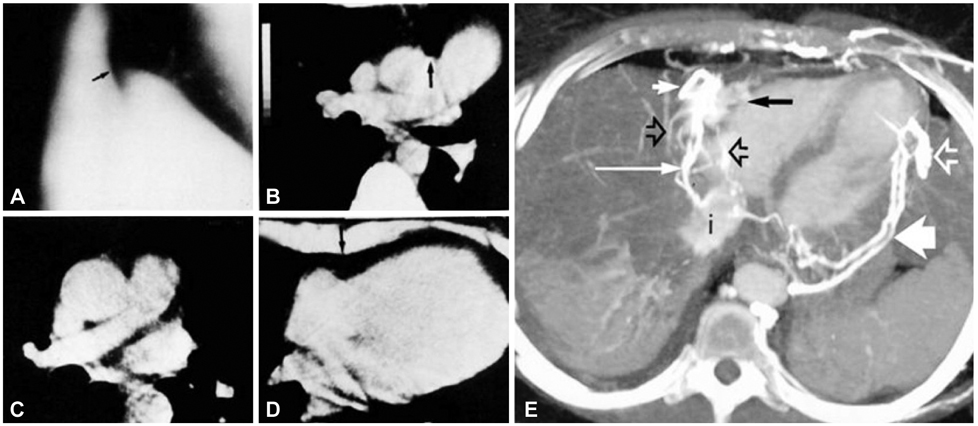
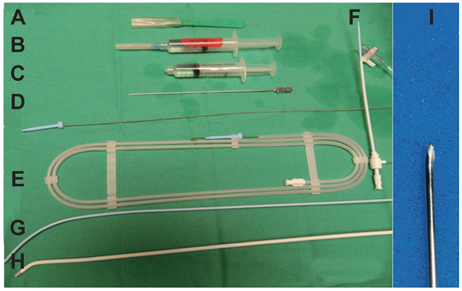
Figure
Reference
-
1. Sosa E, Scanavacca M, d'Avila A, Pilleggi F. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996. 7:531–536.2. Maisch B, Karatolios K. New possibilities of diagnostics and therapy of pericarditis. Internist (Berl). 2008. 49:17–26.3. Braunwald E, Libby P. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 2007. 7 ed. Philadelphia: Saunders.4. Choe YH, Im JG, Park JH, Han MC, Kim CW. The anatomy of the pericardial space: a study in cadavers and patients. AJR Am J Roentgenol. 1987. 149:693–697.5. Groell R, Schaffler GJ, Rienmueller R. Pericardial sinuses and recesses: findings at electrocardiographically triggered electron-beam CT. Radiology. 1999. 212:69–73.6. Levy-Ravetch M, Auh YH, Rubenstein WA, Whalen JP, Kazam E. CT of the pericardial recesses. AJR Am J Roentgenol. 1985. 144:707–714.7. McMurdo KK, Webb WR, von Schulthess GK, Gamsu G. Magnetic resonance imaging of the superior pericardial recesses. AJR Am J Roentgenol. 1985. 145:985–988.8. Vesely TM, Cahill DR. Cross-sectional anatomy of the pericardial sinuses, recesses, and adjacent structures. Surg Radiol Anat. 1986. 8:221–227.9. D'Avila A, Scanavacca M, Sosa E, Ruskin JN, Reddy VY. Pericardial anatomy for the interventional electrophysiologist. J Cardiovasc Electrophysiol. 2003. 14:422–430.10. Chaffanjon P, Brichon PY, Faure C, Favre JJ. Pericardial reflection around the venous aspect of the heart. Surg Radiol Anat. 1997. 19:17–21.11. Shabetai R. Function of the normal pericardium. Clin Cardiol. 1999. 22:1 Suppl 1. I4–I5.12. Miyazaki T, Pride HP, Zipes DP. Prostaglandins in the pericardial fluid modulate neural regulation of cardiac electrophysiological properties. Circ Res. 1990. 66:163–175.13. Verrier RL, Waxman S, Lovett EG, Moreno R. Transatrial access to the normal pericardial space: a novel approach for diagnostic sampling, pericardiocentesis, and therapeutic interventions. Circulation. 1998. 98:2331–2333.14. Seferovic PM, Ristic AD, Maksimovic R, et al. Initial clinical experience with PerDUCER device: promising new tool in the diagnosis and treatment of pericardial disease. Clin Cardiol. 1999. 22:1 Suppl 1. I30–I35.15. Columbus M. Beurlaque N, editor. De re Anatomica. 1559. Vol. 15. Venice:16. Baille M. On the want of a pericardium in the human body. Trans Soc Improve Med Chir Knowl. 1793. 1:91–102.17. Hammoudeh AJ, Kelly ME, Mekhjian H. Congenital total absence of the pericardium: case report of a 72-year-old man and review of the literature. J Thorac Cardiovasc Surg. 1995. 109:805–807.18. Van Son JA, Danielson GK, Schaff HV, Mullany CJ, Julsrud PR, Breen JF. Congenital partial and complete absence of the pericardium. Mayo Clin Proc. 1993. 68:743–747.19. Hano O, Baba T, Hayano M, Yano K. Congenital defect of the left pericardium with sick sinus syndrome. Am Heart J. 1996. 132:1293–1295.20. Lonsky V, Stetina M, Habal P, Markova D. Congenital absence of the left pericardium in combination with left pulmonary artery hypoplasia, right aortic arch, and secundum atrial septal defect. Thorac Cardiovasc Surg. 1992. 40:155–157.21. Lu C, Ridker PM. Echocardiographic diagnosis of congenital absence of the pericardium in a patient with VATER association defects. Clin Cardiol. 1994. 17:503–504.22. Ellis K, Leeds NE, Himmelstein A. Congenital deficiencies in the parietal pericardium: a review with 2 new cases including successful diagnosis by plain roentgenography. Am J Roentgenol Radium Ther Nucl Med. 1959. 82:125–137.23. Baim RS, MacDonald IL, Wise DJ, Lenkei SC. Computed tomography of absent left pericardium. Radiology. 1980. 135:127–128.24. Gutierrez FR, Shackelford GD, McKnight RC, Levitt RG, Hartmann A. Diagnosis of congenital absence of left pericardium by MR imaging. J Comput Assist Tomogr. 1985. 9:551–553.25. Lawler LP, Fishman EK. Pericardial varices: depiction on three-dimensional CT angiography. AJR Am J Roentgenol. 2001. 177:202–204.26. Millward SF, Ramsewak W, Joseph G, Jones B, Zylak CJ. Pericardial varices demonstrated by computed tomography. J Comput Assist Tomogr. 1985. 9:1106–1107.27. Feigin DS, Fenoglio JJ, McAllister HA, Madewell JE. Pericardial cysts: a radiologic-pathologic correlation and review. Radiology. 1977. 125:15–20.28. Inoue S, Fujino S, Tezuka N, et al. Encapsulated pericardial fat necrosis treated by video-assisted thoracic surgery: report of a case. Surg Today. 2000. 30:739–743.29. Stephens DA, Kocab F. Pericardial fat necrosis. J Thorac Cardiovasc Surg. 1988. 95:727–729.30. Sosa E, Scanavacca M, D'Avila A, et al. Endocardial and epicardial ablation guided by nonsurgical transthoracic epicardial mapping to treat recurrent ventricular tachycardia. J Cardiovasc Electrophysiol. 1998. 9:229–239.31. Sosa E, Scanavacca M, d'Avila A, Oliveira F, Ramires JA. Nonsurgical transthoracic epicardial catheter ablation to treat recurrent ventricular tachycardia occurring late after myocardial infarction. J Am Coll Cardiol. 2000. 35:1442–1449.32. D'Avila A, Gutierrez P, Scanavacca M, et al. Effects of radiofrequency pulses delivered in the vicinity of the coronary arteries: implications for nonsurgical transthoracic epicardial catheter ablation to treat ventricular tachycardia. Pacing Clin Electrophysiol. 2002. 25:1488–1495.33. d'Avila A, Houghtaling C, Gutierrez P, et al. Catheter ablation of ventricular epicardial tissue: a comparison of standard and cooled-tip radiofrequency energy. Circulation. 2004. 109:2363–2369.34. Garcia FC, Bazan V, Zado ES, Ren JF, Marchlinski FE. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2009. 120:366–375.35. Schauerte P, Scherlag BJ, Pitha J, et al. Catheter ablation of cardiac autonomic nerves for prevention of vagal atrial fibrillation. Circulation. 2000. 102:2774–2780.36. Zei PC, Stevenson WG. Epicardial catheter mapping and ablation of ventricular tachycardia. Heart Rhythm. 2006. 3:360–363.37. Ayers GM, Rho TH, Ben-David J, Besch HR Jr, Zipes DP. Amiodarone instilled into the canine pericardial sac migrates transmurally to produce electrophysiologic effects and suppress atrial fibrillation. J Cardiovasc Electrophysiol. 1996. 7:713–721.38. Baek SH, Hrabie JA, Keefer LK, et al. Augmentation of intrapericardial nitric oxide level by a prolonged-release nitric oxide donor reduces luminal narrowing after porcine coronary angioplasty. Circulation. 2002. 105:2779–2784.39. Gleason JD, Nguyen KP, Kissinger KV, Manning WJ, Verrier RL. Myocardial drug distribution pattern following intrapericardial delivery: an MRI analysis. J Cardiovasc Magn Reson. 2002. 4:311–316.40. Moreno R, Waxman S, Rowe K, Verrier RL. Intrapericardial beta-adrenergic blockade with esmolol exerts a potent antitachycardic effect without depressing contractility. J Cardiovasc Pharmacol. 2000. 36:722–727.41. Ujhelyi MR, Hadsall KZ, Euler DE, Mehra R. Intrapericardial therapeutics: a pharmacodynamic and pharmacokinetic comparison between pericardial and intravenous procainamide delivery. J Cardiovasc Electrophysiol. 2002. 13:605–611.42. Willerson JT, Igo SR, Yao SK, Ober JC, Macris MP, Ferguson JJ. Localized administration of sodium nitroprusside enhances its protection against platelet aggregation in stenosed and injured coronary arteries. Tex Heart Inst J. 1996. 23:1–8.43. Fei L, Baron AD, Henry DP, Zipes DP. Intrapericardial delivery of L-arginine reduces the increased severity of ventricular arrhythmias during sympathetic stimulation in dogs with acute coronary occlusion: nitric oxide modulates sympathetic effects on ventricular electrophysiological properties. Circulation. 1997. 96:4044–4049.44. Zipes DP. Targeted drug therapy. Circulation. 1990. 81:1139–1141.45. Avitall B, Hare J, Zander G, et al. Iontophoretic transmyocardial drug delivery: a novel approach to antiarrhythmic drug therapy. Circulation. 1992. 85:1582–1593.46. Waxman S, Moreno R, Rowe KA, Verrier RL. Persistent primary coronary dilation induced by transatrial delivery of nitroglycerin into the pericardial space: a novel approach for local cardiac drug delivery. J Am Coll Cardiol. 1999. 33:2073–2077.47. Uchida Y, Yanagisawa-Miwa A, Nakamura F, et al. Angiogenic therapy of acute myocardial infarction by intrapericardial injection of basic fibroblast growth factor and heparin sulfate: an experimental study. Am Heart J. 1995. 130:1182–1188.48. Laham RJ, Hung D, Simons M. Therapeutic myocardial angiogenesis using percutaneous intrapericardial drug delivery. Clin Cardiol. 1999. 22:1 Suppl 1. I6–I9.49. Hermans JJ, van Essen H, Struijker-Boudier HA, Johnson RM, Theeuwes F, Smits JF. Pharmacokinetic advantage of intrapericardially applied substances in the rat. J Pharmacol Exp Ther. 2002. 301:672–678.50. Leithauser B, Park JW. Cardioembolic stroke in atrial fibrillation-rationale for preventive closure of the left atrial appendage. Korean Circ J. 2009. 39:443–458.51. Friedman PA, Asirvatham SJ, Dalegrave C, et al. Percutaneous epicardial left atrial appendage closure: preliminary results of an electrogram guided approach. J Cardiovasc Electrophysiol. 2009. 20:908–915.52. March KL, Woody M, Mehdi K, Zipes DP, Brantly M, Trapnell BC. Efficient in vivo catheter-based pericardial gene transfer mediated by adenoviral vectors. Clin Cardiol. 1999. 22:1 Suppl 1. I23–I29.53. Lau DH, Clausen C, Sosunov EA, et al. Epicardial border zone overexpression of skeletal muscle sodium channel SkM1 normalizes activation, preserves conduction, and suppresses ventricular arrhythmia: an in silico, in vivo, in vitro study. Circulation. 2009. 119:19–27.54. Cho HC, Marban E. Biological therapies for cardiac arrhythmias: can genes and cells replace drugs and devices? Circ Res. 2010. 106:674–685.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Successful Treatment of Cardiac Tamponade Associated with Umbilical Venous Catheter by Pericardiocentesis in Two Neonates: Two Case Reports
- Congenital Partial Left Pericardial Defect
- Pericardial Effusion and Pericardiocentesis: Role of Echocardiography
- A Case of Malignant Pericardial Mesothelioma With Constrictive Pericarditis Physiology Misdiagnosed as Pericardial Metastatic Cancer
- A Case of Behcet's Syndrome with Superior Vena Cava Obstruction and Massive Pericardial Effusion