Cancer Res Treat.
2008 Sep;40(3):127-132.
The Bone Morphogenesis Protein-2 (BMP-2) is Associated with Progression to Metastatic Disease in Gastric Cancer
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Korea University, Seoul, Korea. jskimdr@ naver.com
- 2Division of Hematology/Oncology, College of Medicine, Korea University, Seoul, Korea.
- 3Division of Gastroenterology, College of Medicine, Korea University, Seoul, Korea.
Abstract
- PURPOSE
Bone Morphogenetic Proteins (BMPs) are members of the TGF-beta superfamily and it has been demonstrated that BMPs enhance migration, invasion and metastasis. The purpose of this study was to identify the association between the serum BMP-2 level and the progression status of gastric cancer.
MATERIALS AND METHODS
Fifty-five patients with metastatic gastric cancer (metastatic disease group), six patients with early gastric cancer without lymph node metastasis (the EGC group), and ten healthy control subjects were enrolled in this study. The serum BMP-2 level was quantified by use of a commercially available ELISA kit. In EGC group patients and patients with metastatic disease, whole blood was obtained before endoscopic mucosal resection and before the commencement of a scheduled cycle of systemic chemotherapy, respectively.
RESULTS
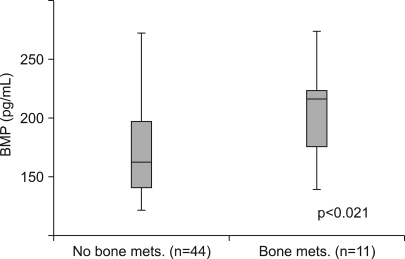
No significant difference in the mean serum BMP-2 levels was observed between the control subjects and the EGC group patients (87.95 pg/ml for the control subjects and 84.50 pg/ml for the EGC group, p=1.0). However, the metastatic disease group patients had a significantly higher level of serum BMP (179.61 pg/ml) than the control subjects and EGC group patients (87.95 pg/ml for the control subjects and 84.50 pg/ml for the EGC group, p<0.0001). Moreover, the mean serum BMP-2 level from patients with a bone metastasis was significantly higher than the mean serum BMP-2 level from patients without a bone metastasis (204.73 pg/ml versus 173.33 pg/ml, p=0.021).
CONCLUSIONS
BMP-2 seems to have a role in progression to metastatic disease in gastric cancer, especially in the late stage of tumorigenesis, including invasion and metastasis. BMP-2 may facilitate bone metastasis in gastric cancer. To confirm these findings, further studies are required with tissue specimens and the use of a cancer cell line.
MeSH Terms
Figure
Reference
-
1. Sampath TK, Maliakal JC, Hauschka PV, Jones WK, Sasak H, Tucker RF, et al. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem. 1992; 267:20352–20362. PMID: 1328198.
Article2. Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996; 10:1580–1594. PMID: 8682290.
Article3. Cao X, Chen D. The BMP signaling and in vivo bone formation. . Gene. 2005; 357:1–8. PMID: 16125875.
Article4. Yang S, Zhong C, Frenkel B, Reddi AH, Roy-Burman P. Diverse biological effect and Smad signaling of bone morphogenetic protein 7 in prostate tumor cells. Cancer Res. 2005; 65:5769–5777. PMID: 15994952.
Article5. Deng H, Makizumi R, Ravikumar TS, Dong H, Yang W, Yang WL. Bone morphogenetic protein-4 is overexpressed in colonic adenocarcinomas and promotes migration and invasion of HCT116 cells. Exp Cell Res. 2007; 313:1033–1044. PMID: 17275810.
Article6. Kleeff J, Maruyama H, Ishiwata T, Sawhney H, Friess H, Buchler MW, et al. Bone morphogenetic protein 2 exerts diverse effects on cell growth in vitro and is expressed in human pancreatic cancer in vivo. Gastroenterology. 1999; 116:1202–1216. PMID: 10220513.
Article7. Kim IY, Kim SJ. Role of bone morphogenetic proteins in transitional cell carcinoma cells. Cancer Lett. 2006; 241:118–123. PMID: 16500023.
Article8. Feeley BT, Krenek L, Liu N, Hsu WK, Gamradt SC, Schwarz EM, et al. Overexpression of noggin inhibits BMP-mediated growth of osteolytic prostate cancer lesions. Bone. 2006; 38:154–166. PMID: 16126463.
Article9. Rothhammer T, Poser I, Soncin F, Bataille F, Moser M, Bosserhoff AK. Bone morphogenic proteins are overexpressed in malignant melanoma and promote cell invasion and migration. Cancer Res. 2005; 65:448–456. PMID: 15695386.10. Langenfeld EM, Calvano SE, Abou-Nukta F, Lowry SF, Amenta P, Langenfeld J. The mature bone morphogenetic protein-2 is aberrantly expressed in non-small cell lung carcinomas and stimulates tumor growth of A549 cells. Carcinogenesis. 2003; 24:1445–1454. PMID: 12819188.
Article11. Arnold SF, Tims E, Mcgrath BE. Identification of bone morphogenetic proteins and their receptors in human breast cancer cell lines: importance of BMP2. Cytokine. 1999; 11:1031–1037. PMID: 10623428.
Article12. Masuda H, Fukabori Y, Nakano K, Takezawa Y, Csuzuki T, Yamanaka H. Increased expression of bone morphogenetic protein-7 in bone metastatic prostate cancer. Prostate. 2003; 54:268–274. PMID: 12539225.
Article13. Yoo YA, Kang MH, Kim JS, Oh SC. Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-beta-mediated activation of the ALK5-Smad 3 pathway. Carcinogenesis. 2008; 29:480–490. PMID: 18174246.14. Hardwick JC, Van Den Brink GR, Bleuming SA, Ballester I, Van Den Brande JM, Keller JJ, et al. Bone morphogenetic protein 2 is expressed by, and acts upon, mature epithelial cells in the colon. Gastroenterology. 2004; 126:111–121. PMID: 14699493.
Article15. Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006; 444:761–765. PMID: 17151667.
Article16. Hallahan AR, Pritchard JI, Chandraratna RA, Ellenbogen RG, Geyer JR, Overland RP, et al. BMP-2 mediates retinoid-induced apoptosis in medulloblastoma cells through a paracrine effect. Nat Med. 2003; 9:1033–1038. PMID: 12872164.
Article17. Kawamura C, Kizaki M, Ikeda Y. Bone morphogenetic protein (BMP)-2 induces apoptosis in human myeloma cells. Leuk Lymphoma. 2002; 43:635–639. PMID: 12002771.
Article18. Kim IY, Lee DH, Lee DK, Kim BC, Kim HT, Leach FS, et al. Decreased expression of bone morphogenetic protein (BMP) receptor type II correlates with insensitivity to BMP-6 in human renal cell carcinoma cells. Clin Cancer Res. 2003; 9:6046–6051. PMID: 14676131.19. Wen XZ, Miyake S, Akiyama Y, Yuasa Y. BMP-2 modulates the proliferation and differentiation of normal and cancerous gastric cells. Biochem Biophys Res Commun. 2004; 316:100–106. PMID: 15003517.
Article20. Langenfeld EM, Kong Y, Langenfeld J. Bone morphogenetic protein 2 stimulation of tumor growth involves the activation of Smad-1/5. Oncogene. 2006; 25:685–692. PMID: 16247476.
Article21. Clement JH, Raida M, Sanger J, Bicknell R, Liu J, Naumann A, et al. Bone morphogenetic protein 2 (BMP-2) induces in vitro invasion and in vivo hormone independent growth of breast carcinoma cells. Int J Oncol. 2005; 27:401–407. PMID: 16010421.
Article22. Montesano R. Bone morphogenetic protein-4 abrogates lumen formation by mammary epithelial cells and promotes invasive growth. Biochem Biophys Res Commun. 2007; 353:817–822. PMID: 17189614.
Article23. Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005; 132:5601–5611. PMID: 16314491.
Article24. Theriault BL, Shepherd TG, Mujoomdar ML, Nachtigal MW. BMP4 induces EMT and Rho GTPase activation in human ovarian cancer cells. Carcinogenesis. 2007; 28:1153–1162. PMID: 17272306.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy for Metastatic Gastric Cancer
- The Analysis of Bone regenerative effect with carriers of bone morphogenetic protein in rat calvarial defects
- Enhanced Bone Regeneration by Bone Morphogenetic Protein-2 after Pretreatment with Low-Intensity Pulsed Ultrasound in Distraction Osteogenesis
- Comparative Study of 3-Dimensional-Printed Poly-L-Lactic Acid/Bone Morphogenetic Protein (BMP)/Collagen Bone Substitute and Commercial Hydroxyapatite/BMP for Bone Regeneration Efficacy Using a Mouse Calvarial Model
- Effects Of Chitosan On Human Osteoblasts