J Korean Med Sci.
2014 Sep;29(9):1278-1286. 10.3346/jkms.2014.29.9.1278.
Influence of Propofol and Fentanyl on Deep Brain Stimulation of the Subthalamic Nucleus
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University College of Medicine, Seoul, Korea. paeksh@snu.ac.kr
- 2Medical Device Development Center, Osong Medical Innovation Foundation, Cheongwon, Korea.
- 3Department of Neurology, Seoul National University College of Medicine, Seoul, Korea.
- 4Medical Imaging Laboratory and CyberMed, Inc., Seoul, Korea.
- 5Department of Medical Engineering, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Biomedical Engineering, Hanyang University, Seoul, Korea.
- 7Department of Anaesthesiology and Pain Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 8Ischemic/Hypoxic Disease Institute, Seoul National University College of Medicine, Seoul, Korea.
- 9Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 10Clinical Research Institute, Seoul National University Hospital, Seoul, Korea.
- KMID: 1794608
- DOI: http://doi.org/10.3346/jkms.2014.29.9.1278
Abstract
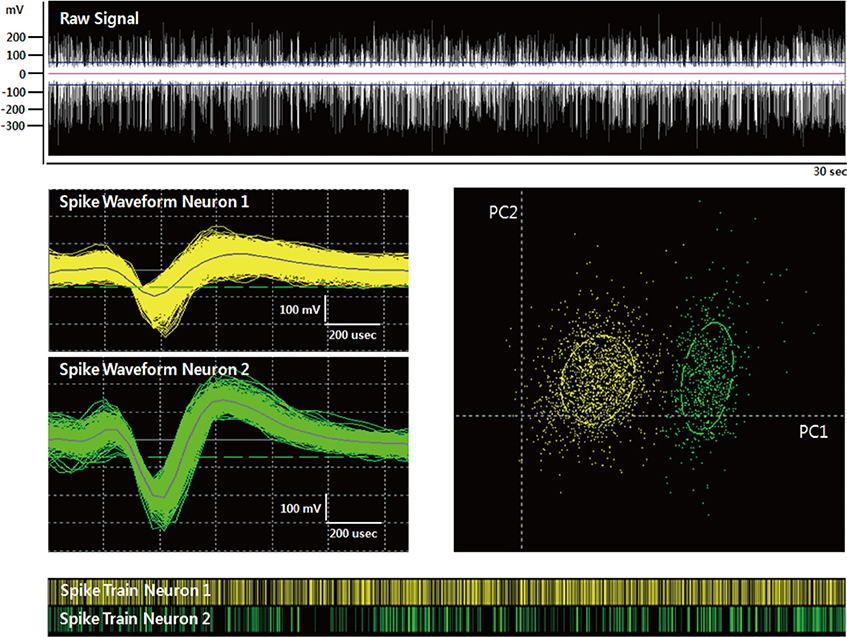
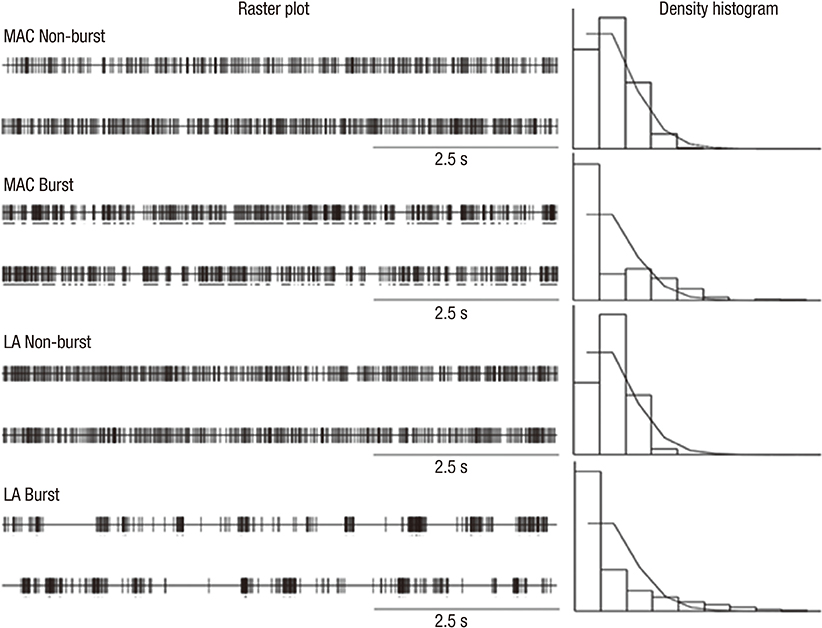
- We investigated the effect of propofol and fentanyl on microelectrode recording (MER) and its clinical applicability during subthalamic nucleus (STN) deep brain stimulation (DBS) surgery. We analyzed 8 patients with Parkinson's disease, underwent bilateral STN DBS with MER. Their left sides were done under awake and then their right sides were done with a continuous infusion of propofol and fentanyl under local anesthesia. The electrode position was evaluated by preoperative MRI and postoperative CT. The clinical outcomes were assessed at six months after surgery. We isolated single unit activities from the left and the right side MERs. There was no significant difference in the mean firing rate between the left side MERs (38.7+/-16.8 spikes/sec, n=78) and the right side MERs (35.5+/-17.2 spikes/sec, n=66). The bursting pattern of spikes was more frequently observed in the right STN than in the left STN. All the electrode positions were within the STNs on both sides and the off-time Unified Parkinson's Disease Rating Scale part III scores at six months after surgery decreased by 67% of the preoperative level. In this study, a continuous infusion of propofol and fentanyl did not significantly interfere with the MER signals from the STN. The results of this study suggest that propofol and fentanyl can be used for STN DBS in patients with advanced Parkinson's disease improving the overall experience of the patients.
Keyword
MeSH Terms
-
Aged
Anesthetics, Intravenous/*pharmacology
*Deep Brain Stimulation
Electrodes, Implanted
Female
Fentanyl/*pharmacology
Humans
Magnetic Resonance Imaging
Male
Microelectrodes
Middle Aged
Parkinson Disease/*prevention & control
Propofol/*pharmacology
Severity of Illness Index
Subthalamic Nucleus/*drug effects/physiology
Tomography, X-Ray Computed
Anesthetics, Intravenous
Fentanyl
Propofol
Figure
Cited by 1 articles
-
Practical considerations and nuances in anesthesia for patients undergoing deep brain stimulation implantation surgery
Danielle Teresa Scharpf, Mayur Sharma, Milind Deogaonkar, Ali Rezai, Sergio D. Bergese
Korean J Anesthesiol. 2015;68(4):332-339. doi: 10.4097/kjae.2015.68.4.332.
Reference
-
1. Deep-Brain Stimulation for Parkinson's Disease Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. N Engl J Med. 2001; 345:956–963.2. Benabid AL. Deep brain stimulation for Parkinson's disease. Curr Opin Neurobiol. 2003; 13:696–706.3. Hutchison WD, Allan RJ, Opitz H, Levy R, Dostrovsky JO, Lang AE, Lozano AM. Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson's disease. Ann Neurol. 1998; 44:622–628.4. Hertel F, Züchner M, Weimar I, Gemmar P, Noll B, Bettag M, Decker C. Implantation of electrodes for deep brain stimulation of the subthalamic nucleus in advanced Parkinson's disease with the aid of intraoperative microrecording under general anesthesia. Neurosurgery. 2006; 59:E1138.5. Lefaucheur JP, Gurruchaga JM, Pollin B, von Raison F, Mohsen N, Shin M, Ménard-Lefaucheur I, Oshino S, Kishima H, Fénelon G, et al. Outcome of bilateral subthalamic nucleus stimulation in the treatment of Parkinson's disease: correlation with intra-operative multi-unit recordings but not with the type of anaesthesia. Eur Neurol. 2008; 60:186–199.6. Maltête D, Navarro S, Welter ML, Roche S, Bonnet AM, Houeto JL, Mesnage V, Pidoux B, Dormont D, Cornu P, et al. Subthalamic stimulation in Parkinson disease: with or without anesthesia? Arch Neurol. 2004; 61:390–392.7. Paek SH, Kim HJ, Yoon JY, Heo JH, Kim C, Kim MR, Lim YH, Kim KR, Kim JW, Han JH, et al. Fusion image-based programming after subthalamic nucleus deep brain stimulation. World Neurosurg. 2011; 75:517–524.8. Schaltenbrand G, Wharen W. Atlas of stereotaxy of the human brain. New York: Thieme;1998.9. Harries AM, Kausar J, Roberts SA, Mocroft AP, Hodson JA, Pall HS, Mitchell RD. Deep brain stimulation of the subthalamic nucleus for advanced Parkinson disease using general anesthesia: long-term results. J Neurosurg. 2012; 116:107–113.10. Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987; 50:344–346.11. Khan MF, Mewes K, Gross RE, Skrinjar O. Assessment of brain shift related to deep brain stimulation surgery. Stereotact Funct Neurosurg. 2008; 86:44–53.12. Martinez-Santiesteban FM, Swanson SD, Noll DC, Anderson DJ. Magnetic field perturbation of neural recording and stimulating microelectrodes. Phys Med Biol. 2007; 52:2073–2088.13. Miyagi Y, Shima F, Sasaki T. Brain shift: an error factor during implantation of deep brain stimulation electrodes. J Neurosurg. 2007; 107:989–997.14. Benarroch EE. Subthalamic nucleus and its connections: anatomic substrate for the network effects of deep brain stimulation. Neurology. 2008; 70:1991–1995.15. Deiner S, Hagen J. Parkinson's disease and deep brain stimulator placement. Anesthesiol Clin. 2009; 27:391–415.16. Duque P, Mateo O, Ruiz F, de Viloria JG, Contreras A, Grandas F. Intraoperative microrecording under general anaesthesia with bispectral analysis monitoring in a case of deep brain stimulation surgery for Parkinson's disease. Eur J Neurol. 2008; 15:e76–e77.17. Lin SH, Chen TY, Lin SZ, Shyr MH, Chou YC, Hsieh WA, Tsai ST, Chen SY. Subthalamic deep brain stimulation after anesthetic inhalation in Parkinson disease: a preliminary study. J Neurosurg. 2008; 109:238–244.18. Yamada K, Goto S, Kuratsu J, Matsuzaki K, Tamura T, Nagahiro S, Murase N, Shimazu H, Kaji R. Stereotactic surgery for subthalamic nucleus stimulation under general anesthesia: a retrospective evaluation of Japanese patients with Parkinson's disease. Parkinsonism Relat Disord. 2007; 13:101–107.19. Benazzouz A, Breit S, Koudsie A, Pollak P, Krack P, Benabid AL. Intraoperative microrecordings of the subthalamic nucleus in Parkinson's disease. Mov Disord. 2002; 17:S145–S149.20. Maciver MB, Bronte-Stewart HM, Henderson JM, Jaffe RA, Brock-Utne JG. Human subthalamic neuron spiking exhibits subtle responses to sedatives. Anesthesiology. 2011; 115:254–264.21. Magnin M, Morel A, Jeanmonod D. Single-unit analysis of the pallidum, thalamus and subthalamic nucleus in parkinsonian patients. Neuroscience. 2000; 96:549–564.22. Rodriguez-Oroz MC, Rodriguez M, Guridi J, Mewes K, Chockkman V, Vitek J, DeLong MR, Obeso JA. The subthalamic nucleus in Parkinson's disease: somatotopic organization and physiological characteristics. Brain. 2001; 124:1777–1790.23. Schrock LE, Ostrem JL, Turner RS, Shimamoto SA, Starr PA. The subthalamic nucleus in primary dystonia: single-unit discharge characteristics. J Neurophysiol. 2009; 102:3740–3752.24. Steigerwald F, Pötter M, Herzog J, Pinsker M, Kopper F, Mehdorn H, Deuschl G, Volkmann J. Neuronal activity of the human subthalamic nucleus in the parkinsonian and nonparkinsonian state. J Neurophysiol. 2008; 100:2515–2524.25. Weinberger M, Mahant N, Hutchison WD, Lozano AM, Moro E, Hodaie M, Lang AE, Dostrovsky JO. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson's disease. J Neurophysiol. 2006; 96:3248–3256.26. Hutchison WD, Lang AE, Dostrovsky JO, Lozano AM. Pallidal neuronal activity: implications for models of dystonia. Ann Neurol. 2003; 53:480–488.27. Anderson BJ, Marks PV, Futter ME. Propofol: contrasting effects in movement disorders. Br J Neurosurg. 1994; 8:387–388.28. Böhmdorfer W, Schwarzinger P, Binder S, Sporn P. Temporary suppression of tremor by remifentanil in a patient with Parkinson's disease during cataract extraction under local anesthesia. Anaesthesist. 2003; 52:795–797.29. Velly LJ, Rey MF, Bruder NJ, Gouvitsos FA, Witjas T, Regis JM, Peragut JC, Gouin FM. Differential dynamic of action on cortical and subcortical structures of anesthetic agents during induction of anesthesia. Anesthesiology. 2007; 107:202–212.30. Venkatraghavan L, Luciano M, Manninen P. Review article: anesthetic management of patients undergoing deep brain stimulator insertion. Anesth Analg. 2010; 110:1138–1145.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Deep Brain Stimulation of the Subthalamic and Pedunculopontine Nucleus in a Patient with Parkinson's Disease
- Deep Brain Stimulation of the Subthalamic Area for Dystonic Tremor
- Deep Brain Stimulation for the Treatment of Medically Intractable Epilepsy: a Review on Clinical Application
- Microelectrode Recording-Guided Deep Brain Stimulation in Patients with Movement Disorders
- Posterior subthalamic area deep brain stimulation for recurrent tremor after ventral intermediate nucleus thalamotomy: a case report