Yonsei Med J.
2013 Sep;54(5):1234-1240. 10.3349/ymj.2013.54.5.1234.
Posaconazole Treatment in Korea: Single-Center Experience Over 5 Years
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. symonlee@catholic.ac.kr
- 2Division of Infectious Diseases, Department of Internal Medicine, National Health Insurance Corporation Ilsan Hospital, Goyang, Korea.
- 3Department of Life Sciences, Pohang University of Science and Technology, Pohang, Korea.
- 4Catholic Blood and Marrow Transplantation Center, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 1793173
- DOI: http://doi.org/10.3349/ymj.2013.54.5.1234
Abstract
- PURPOSE
Posaconazole is a second-generation triazole with a broad spectrum. However, there is a lack of data to support a significant role for posaconazole in the treatment of invasive fungal infection (IFI), especially in Korea. Until recently, posaconazole was available only through the Korean Orphan Drug Center. This study was designed to review the use of posaconazole at a single-center in Korea.
MATERIALS AND METHODS
Data from patients who received posaconazole treatment at Catholic Blood and Marrow Transplantation Center were retrospectively reviewed between January 2007 and September 2012.
RESULTS
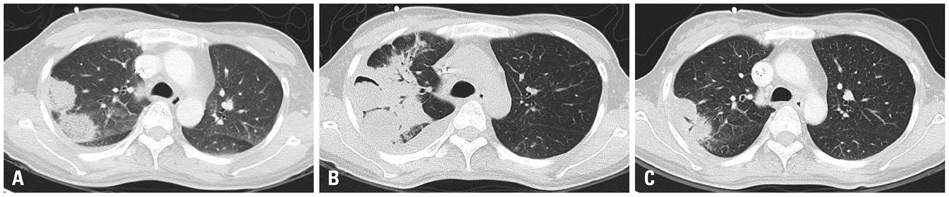
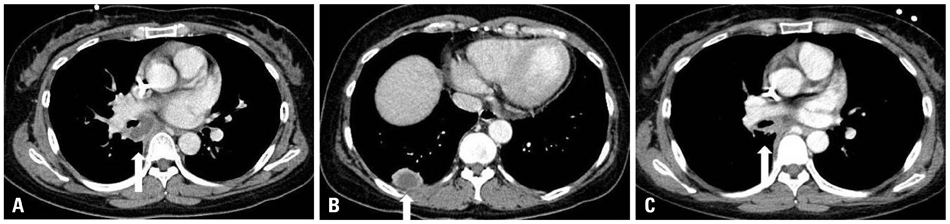
A total of 11 cases (3 males and 8 females, median age 52 years) received posaconazole. Five patients were given the drug for mucormycosis, two for invasive aspergillosis, and four for unspecified IFI for which galactomannan (GM) assays were negative. The treatment duration ranged from 4-250 days. Three patients received posaconazole for management refractory IFI, two for intolerance of previous antifungal therapy, and six for long-term maintenance treatment. The overall successful response rate to posaconazole was 55% (six of eleven patients). Five of eleven patients died during the study period. However, only one death was attributed to the progression of IFI. None of the patients discontinued posaconazole therapy due to adverse events.
CONCLUSION
Posaconazole is an attractive oral antifungal agent for salvage treatment of IFI, particularly upon diagnosis of mucormycosis or in cases in which mucormycosis cannot be ruled out due to a negative GM.
MeSH Terms
Figure
Reference
-
1. Mullane K, Toor AA, Kalnicky C, Rodriguez T, Klein J, Stiff P. Posaconazole salvage therapy allows successful allogeneic hematopoietic stem cell transplantation in patients with refractory invasive mold infections. Transpl Infect Dis. 2007; 9:89–96.
Article2. Rachwalski EJ, Wieczorkiewicz JT, Scheetz MH. Posaconazole: an oral triazole with an extended spectrum of activity. Ann Pharmacother. 2008; 42:1429–1438.
Article3. Greenberg RN, Mullane K, van Burik JA, Raad I, Abzug MJ, Anstead G, et al. Posaconazole as salvage therapy for zygomycosis. Antimicrob Agents Chemother. 2006; 50:126–133.
Article4. Nagappan V, Deresinski S. Reviews of anti-infective agents: posaconazole: a broad-spectrum triazole antifungal agent. Clin Infect Dis. 2007; 45:1610–1617.5. Morris MI. Posaconazole: a new oral antifungal agent with an expanded spectrum of activity. Am J Health Syst Pharm. 2009; 66:225–236.
Article6. De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008; 46:1813–1821.
Article7. Segal BH, Herbrecht R, Stevens DA, Ostrosky-Zeichner L, Sobel J, Viscoli C, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis. 2008; 47:674–683.
Article8. Raad II, Hachem RY, Herbrecht R, Graybill JR, Hare R, Corcoran G, et al. Posaconazole as salvage treatment for invasive fusariosis in patients with underlying hematologic malignancy and other conditions. Clin Infect Dis. 2006; 42:1398–1403.
Article9. Lee DG, Kim SH, Kim SY, Kim CJ, Park WB, Song YG, et al. Evidence-based guidelines for empirical therapy of neutropenic fever in Korea. Korean J Intern Med. 2011; 26:220–252.
Article10. Common terminology criteria for adverse events (CTCAE) v4.0. National Cancer Institute;2009.11. Yoon YK, Kim MJ, Chung YG, Shin IY. Successful treatment of a case with rhino-orbital-cerebral mucormycosis by the combination of neurosurgical intervention and the sequential use of amphotericin B and posaconazole. J Korean Neurosurg Soc. 2010; 47:74–77.
Article12. Kim WJ, Han SY, Nam YH, Kim JM, Ahn HB, Kim SJ, et al. A case of successful posaconazole salvage therapy for rhinocerebral mucormycosis after failure of amphotericin B. Korean J Med. 2010; 79:587–591.13. Alastruey-Izquierdo A, Castelli MV, Cuesta I, Zaragoza O, Monzón A, Mellado E, et al. In vitro activity of antifungals against Zygomycetes. Clin Microbiol Infect. 2009; 15:Suppl 5. 71–76.
Article14. Cornely OA, Vehreschild JJ, Rüping MJ. Current experience in treating invasive zygomycosis with posaconazole. Clin Microbiol Infect. 2009; 15:Suppl 5. 77–81.
Article15. Almyroudis NG, Sutton DA, Fothergill AW, Rinaldi MG, Kusne S. In vitro susceptibilities of 217 clinical isolates of zygomycetes to conventional and new antifungal agents. Antimicrob Agents Chemother. 2007; 51:2587–2590.
Article16. Raad II, Hanna HA, Boktour M, Jiang Y, Torres HA, Afif C, et al. Novel antifungal agents as salvage therapy for invasive aspergillosis in patients with hematologic malignancies: posaconazole compared with high-dose lipid formulations of amphotericin B alone or in combination with caspofungin. Leukemia. 2008; 22:496–503.
Article17. Traunmüller F, Popovic M, Konz KH, Smolle-Jüttner FM, Joukhadar C. Efficacy and safety of current drug therapies for invasive aspergillosis. Pharmacology. 2011; 88:213–224.
Article18. Walsh TJ, Raad I, Patterson TF, Chandrasekar P, Donowitz GR, Graybill R, et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis. 2007; 44:2–12.
Article19. Greer ND. Posaconazole (Noxafil): a new triazole antifungal agent. Proc (Bayl Univ Med Cent). 2007; 20:188–196.
Article20. van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006; 42:e61–e65.
Article21. Krishnan-Natesan S, Manavathu EK, Alangaden GJ, Chandrasekar PH. A comparison of the fungicidal activity of amphotericin B and posaconazole against Zygomycetes in vitro. Diagn Microbiol Infect Dis. 2009; 63:361–364.
Article22. Kontoyiannis DP. Invasive mycoses: strategies for effective management. Am J Med. 2012; 125:1 Suppl. S25–S38.
Article23. Bhattacharya M, Rajeshwari K, Dhingra B. Posaconazole. J Postgrad Med. 2010; 56:163–167.
Article24. Raad II, Graybill JR, Bustamante AB, Cornely OA, Gaona-Flores V, Afif C, et al. Safety of long-term oral posaconazole use in the treatment of refractory invasive fungal infections. Clin Infect Dis. 2006; 42:1726–1734.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Posaconazole versus Itraconazole as Prophylactic Antifungal Agents during Induction Chemotherapy for Acute Myeloid Leukemia: A Real-World Single Center Comparison
- Fatal Breakthrough Mucormycosis in an Acute Myelogenous Leukemia Patient while on Posaconazole Prophylaxis
- Therapeutic Use of Posaconazole for Cutaneous Purpureocillium lilacinum Infection Refractory to Itraconazole
- A Case of Rhino-Orbital-Cerebral Mucormycosis Successfully Treated by Surgical Treatment and Posaconazole
- A case of successful posaconazole salvage therapy for rhinocerebral mucormycosis after failure of amphotericin B