J Korean Med Sci.
2013 Sep;28(9):1394-1398. 10.3346/jkms.2013.28.9.1394.
An Infant with Prenatally Diagnosed Congenital Anaplastic Astrocytoma Who Remains Disease-Free after Proton Therapy
- Affiliations
-
- 1Joy Pediatric Clinic, Seoul, Korea.
- 2Center for Pediatric Oncology, National Cancer Center, Goyang, Korea. hjpark@ncc.re.kr
- 3Neuro-Oncology Clinic, National Cancer Center, Goyang, Korea.
- 4Proton Therapy Center, National Cancer Center, Goyang, Korea.
- 5Department of Diagnostic Radiology, National Cancer Center, Goyang, Korea.
- 6Department of Pediatrics, Keimyung University School of Medicine, Daegu, Korea.
- 7Department of Neurosurgery, Keimyung University School of Medicine, Daegu, Korea.
- KMID: 1793059
- DOI: http://doi.org/10.3346/jkms.2013.28.9.1394
Abstract
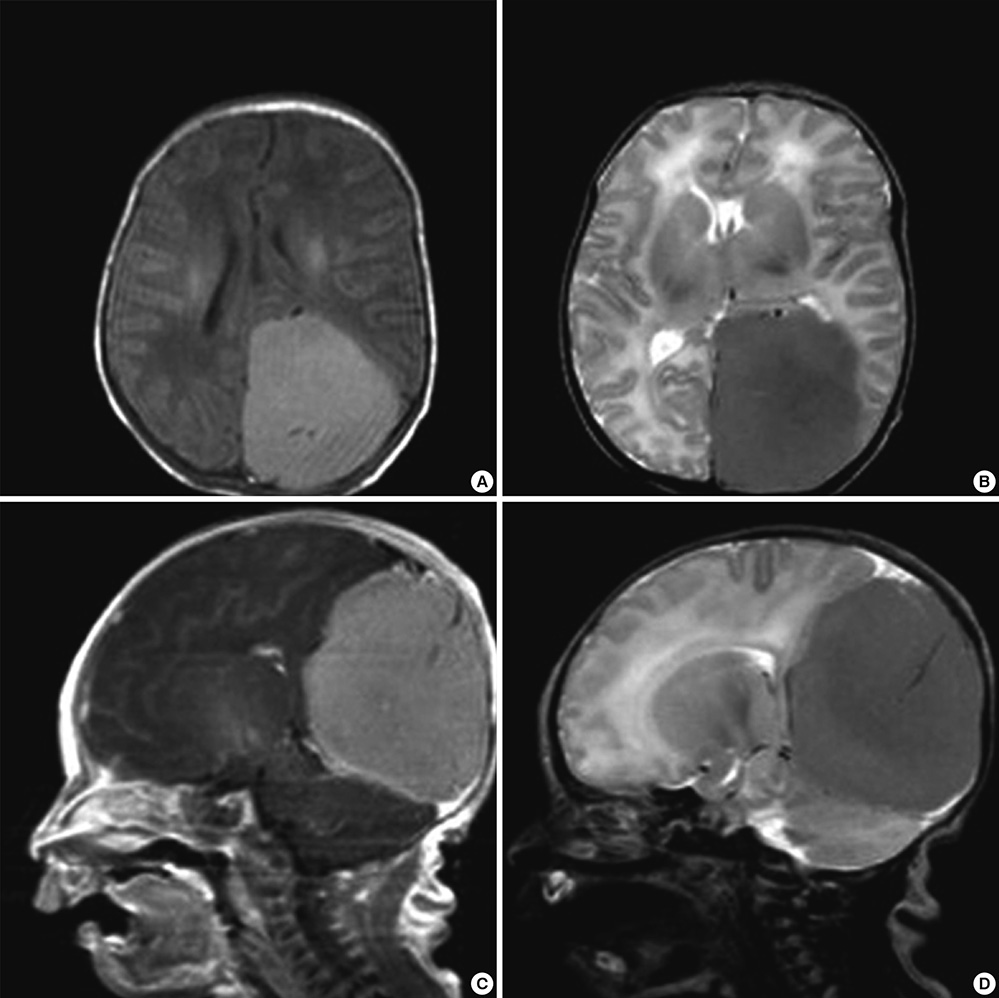
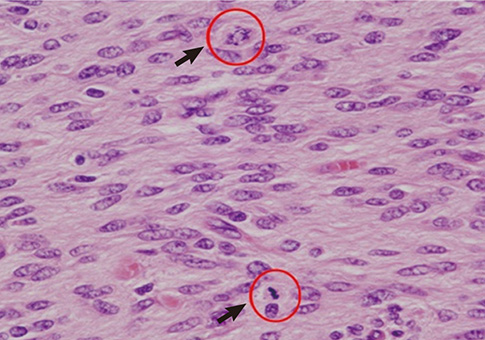
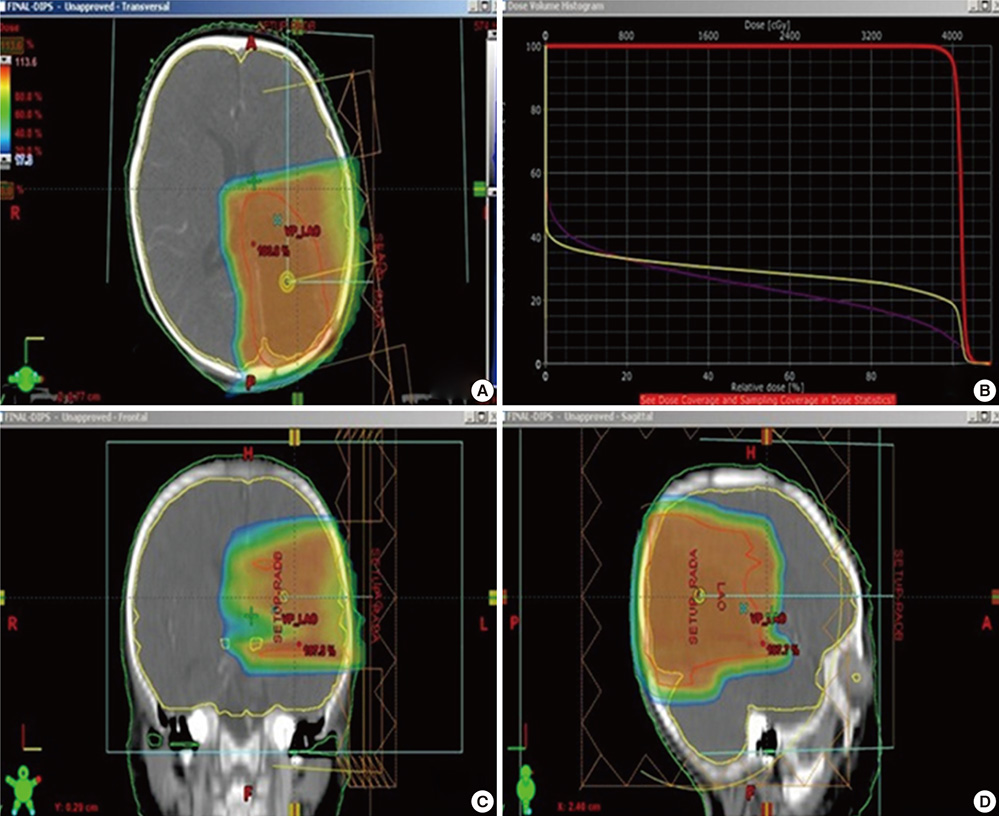
- The authors present a rare of prenatally diagnosed congenital anaplastic astrocytoma. A 9-month-old boy had three recurrences despite two surgical resections and various chemotherapeutic regimens. He underwent the 3rd gross tumor removal at 11 months of age, followed by proton therapy, and now he remains disease-free for 3 yr without a significant neurocognitive dysfunction. This is the 1st case of a pediatric tumor treated by proton therapy in Korea, and proton therapy may be a treatment of choice for a congenital anaplastic astrocytoma in infants and young children, considering limitation of radiation therapy.
MeSH Terms
Figure
Reference
-
1. Balestrini MR, Micheli R, Giordano L, Lasio G, Giombini S. Brain tumors with symptomatic onset in the first two years of life. Childs Nerv Syst. 1994; 10:104–110.2. Jooma R, Kendall BE. Intracranial tumours in the first year of life. Neuroradiology. 1982; 23:267–274.3. Chung SN, Rosemond RL, Graham D. Prenatal diagnosis of a fetal intracranial tumor. J Ultrasound Med. 1998; 17:521–523.4. Oi S, Kokunai T, Matsumoto S. Congenital brain tumors in Japan (ISPN Cooperative Study): specific clinical features in neonates. Childs Nerv Syst. 1990; 6:86–91.5. Buetow PC, Smirniotopoulos JG, Done S. Congenital brain tumors: a review of 45 cases. AJR Am J Roentgenol. 1990; 155:587–593.6. Campbell JW, Pollack IF, Martinez AJ, Shultz B. High-grade astrocytomas in children: radiologically complete resection is associated with an excellent long-term prognosis. Neurosurgery. 1996; 38:258–264.7. Finlay JL, Boyett JM, Yates AJ, Wisoff JH, Milstein JM, Geyer JR, Bertolone SJ, McGuire P, Cherlow JM, Tefft M, et al. Randomized phase III trial in childhood high-grade astrocytoma comparing vincristine, lomustine, and prednisone with the eight-drugs-in-1-day regimen: Childrens Cancer Group. J Clin Oncol. 1995; 13:112–123.8. Heideman RL, Kuttesch J Jr, Gajjar AJ, Walter AW, Jenkins JJ, Li Y, Sanford RA, Kun LE. Supratentorial malignant gliomas in childhood: a single institution perspective. Cancer. 1997; 80:497–504.9. Sposto R, Ertel IJ, Jenkin RD, Boesel CP, Venes JL, Ortega JA, Evans AE, Wara W, Hammond D. The effectiveness of chemotherapy for treatment of high grade astrocytoma in children: results of a randomized trial: a report from the Childrens Cancer Study Group. J Neurooncol. 1989; 7:165–177.10. Wisoff JH, Boyett JM, Berger MS, Brant C, Li H, Yates AJ, McGuire-Cullen P, Turski PA, Sutton LN, Allen JC, et al. Current neurosurgical management and the impact of the extent of resection in the treatment of malignant gliomas of childhood: a report of the Children's Cancer Group trial no. CCG-945. J Neurosurg. 1998; 89:52–59.11. Finlay JL, Goldman S, Wong MC, Cairo M, Garvin J, August C, Cohen BH, Stanley P, Zimmerman RA, Bostrom B, et al. Pilot study of high-dose thiotepa and etoposide with autologous bone marrow rescue in children and young adults with recurrent CNS tumors: the Children's Cancer Group. J Clin Oncol. 1996; 14:2495–2503.12. Reimers TS, Ehrenfels S, Mortensen EL, Schmiegelow M, Sønderkaer S, Carstensen H, Schmiegelow K, Müller J. Cognitive deficits in long-term survivors of childhood brain tumors: identification of predictive factors. Med Pediatr Oncol. 2003; 40:26–34.13. Robertson PL, Muraszko KM, Holmes EJ, Sposto R, Packer RJ, Gajjar A, Dias MS, Allen JC. Children's Oncology Group. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children's Oncology Group. J Neurosurg. 2006; 105:444–451.14. Sønderkaer S, Schmiegelow M, Carstensen H, Nielsen LB, Müller J, Schmiegelow K. Long-term neurological outcome of childhood brain tumors treated by surgery only. J Clin Oncol. 2003; 21:1347–1351.15. Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973; 48:757–767.16. Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009; 27:3691–3697.17. Fuss M, Hug EB, Schaefer RA, Nevinny-Stickel M, Miller DW, Slater JM, Slater JD. Proton radiation therapy (PRT) for pediatric optic pathway gliomas: comparison with 3D planned conventional photons and a standard photon technique. Int J Radiat Oncol Biol Phys. 1999; 45:1117–1126.18. Lee CT, Bilton SD, Famiglietti RM, Riley BA, Mahajan A, Chang EL, Maor MH, Woo SY, Cox JD, Smith AR. Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: how do protons compare with other conformal techniques? Int J Radiat Oncol Biol Phys. 2005; 63:362–372.19. Mu X, Björk-Eriksson T, Nill S, Oelfke U, Johansson KA, Gagliardi G, Johansson L, Karlsson M, Zackrisson DB. Does electron and proton therapy reduce the risk of radiation induced cancer after spinal irradiation for childhood medulloblastoma? a comparative treatment planning study. Acta Oncol. 2005; 44:554–562.20. Weber DC, Trofimov AV, Delaney TF, Bortfeld T. A treatment planning comparison of intensity modulated photon and proton therapy for paraspinal sarcomas. Int J Radiat Oncol Biol Phys. 2004; 58:1596–1606.