J Korean Med Sci.
2013 Sep;28(9):1302-1306. 10.3346/jkms.2013.28.9.1302.
Impact of Interleukin-10 Gene Polymorphisms on Survival in Patients with Colorectal Cancer
- Affiliations
-
- 1Department of Colorectal Surgery, China Medical University Hospital, Taichung, Taiwan.
- 2Division of Colorectal Surgery, Department of Surgery, Chung Shan Medical University Hospital, Taichung, Taiwan.
- 3Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan.
- 4Department of Obstetrics and Gynecology, China Medical University Hospital, Taichung, Taiwan.
- 5Department of Pharmacy, China Medical University, Taichung, Taiwan. bao@mail.cmu.edu.tw
- 6Department of Chinese Medicine Resources, China Medical University, Taichung, Taiwan.
- 7Department of Occupational Safety and Health, China Medical University, Taichung, Taiwan.
- 8Sex Hormone Research Center, China Medical University Hospital, Taichung, Taiwan.
- KMID: 1793044
- DOI: http://doi.org/10.3346/jkms.2013.28.9.1302
Abstract
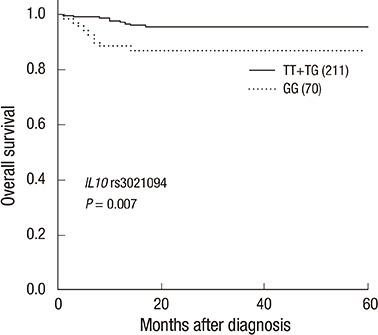
- Chronic inflammation is thought to be the leading cause of colorectal cancer, and interleukin-10 (IL10) has been identified as a potent immunomodulatory cytokine that regulates inflammatory responses in the gastrointestinal tract. Although several single nucleotide polymorphisms (SNPs) in IL10 have been associated with the risk of colorectal cancer, their prognostic significance has not been determined. Two hundred and eighty-two colorectal cancer patients were genotyped for two candidate cancer-associated SNPs in IL10. The associations of these SNPs with distant metastasis-free survival and overall survival were evaluated by Kaplan-Meier analysis and Cox regression model. The minor homozygote GG genotype of IL10 rs3021094 was significantly associated with a 3.30-fold higher risk of death compared with the TT+TG genotypes (P=0.011). The patients with IL10 rs3021094 GG genotype also had a poorer overall survival in Kaplan-Meier analysis (log-rank P=0.007) and in multivariate Cox regression model (P=0.044) adjusting for age, gender, carcinoembryonic antigen levels, tumor differentiation, stage, lymphovascular invasion, and perineural invasion. In conclusion, our results suggest that IL10 rs3021094 might be a valuable prognostic biomarker for colorectal cancer patients.
MeSH Terms
-
Aged
Alleles
Carcinoembryonic Antigen/blood
Cell Differentiation
Colorectal Neoplasms/*genetics/mortality/pathology
Female
Genotype
Homozygote
Humans
Interleukin-10/*genetics
Kaplan-Meier Estimate
Lymphatic Metastasis
Male
Middle Aged
Neoplasm Staging
*Polymorphism, Single Nucleotide
Regression Analysis
Tumor Markers, Biological/genetics
Carcinoembryonic Antigen
Interleukin-10
Tumor Markers, Biological
Figure
Reference
-
1. Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002; 20:495–549.2. Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D, Rickman BH, Rogers AB, Moroski-Erkul CA, McFaline JL, et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest. 2008; 118:2516–2525.3. Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008; 14:378–389.4. Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006; 6:836–848.5. Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001; 19:683–765.6. Berg DJ, Davidson N, Kühn R, Müller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996; 98:1010–1020.7. Gunter MJ, Canzian F, Landi S, Chanock SJ, Sinha R, Rothman N. Inflammation-related gene polymorphisms and colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2006; 15:1126–1131.8. Macarthur M, Sharp L, Hold GL, Little J, El-Omar EM. The role of cytokine gene polymorphisms in colorectal cancer and their interaction with aspirin use in the northeast of Scotland. Cancer Epidemiol Biomarkers Prev. 2005; 14:1613–1618.9. Tsilidis KK, Helzlsouer KJ, Smith MW, Grinberg V, Hoffman-Bolton J, Clipp SL, Visvanathan K, Platz EA. Association of common polymorphisms in IL10, and in other genes related to inflammatory response and obesity with colorectal cancer. Cancer Causes Control. 2009; 20:1739–1751.10. Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, Belmont JW, Boudreau A, Hardenbol P, Leal SM, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007; 449:851–861.11. Bao BY, Pao JB, Huang CN, Pu YS, Chang TY, Lan YH, Lu TL, Lee HZ, Chen LM, Ting WC, et al. Significant associations of prostate cancer susceptibility variants with survival in patients treated with androgen-deprivation therapy. Int J Cancer. 2012; 130:876–884.12. Bao BY, Pao JB, Huang CN, Pu YS, Chang TY, Lan YH, Lu TL, Lee HZ, Juang SH, Chen LM, et al. Polymorphisms inside microRNAs and microRNA target sites predict clinical outcomes in prostate cancer patients receiving androgen-deprivation therapy. Clin Cancer Res. 2011; 17:928–936.13. Bao BY, Pao JB, Lin VC, Huang CN, Chang TY, Lan YH, Lu TL, Lee HZ, Chen LM, Ting WC, et al. Individual and cumulative association of prostate cancer susceptibility variants with clinicopathologic characteristics of the disease. Clin Chim Acta. 2010; 411:1232–1237.14. Huang CN, Huang SP, Pao JB, Chang TY, Lan YH, Lu TL, Lee HZ, Juang SH, Wu PP, Pu YS, et al. Genetic polymorphisms in androgen receptor-binding sites predict survival in prostate cancer patients receiving androgen-deprivation therapy. Ann Oncol. 2012; 23:707–713.15. Huang CN, Huang SP, Pao JB, Hour TC, Chang TY, Lan YH, Lu TL, Lee HZ, Juang SH, Wu PP, et al. Genetic polymorphisms in oestrogen receptor-binding sites affect clinical outcomes in patients with prostate cancer receiving androgen-deprivation therapy. J Intern Med. 2012; 271:499–509.16. Huang SP, Lan YH, Lu TL, Pao JB, Chang TY, Lee HZ, Yang WH, Hsieh CJ, Chen LM, Huang LC, et al. Clinical significance of runt-related transcription factor 1 polymorphism in prostate cancer. BJU Int. 2011; 107:486–492.17. Wanebo HJ, Rao B, Pinsky CM, Hoffman RG, Stearns M, Schwartz MK, Oettgen HF. Preoperative carcinoembryonic antigen level as a prognostic indicator in colorectal cancer. N Engl J Med. 1978; 299:448–451.18. Franke A, Balschun T, Karlsen TH, Sventoraityte J, Nikolaus S, Mayr G, Domingues FS, Albrecht M, Nothnagel M, Ellinghaus D, et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008; 40:1319–1323.19. Xu Z, Taylor JA. SNPinfo: integrating GWAS and candidate gene information into functional SNP selection for genetic association studies. Nucleic Acids Res. 2009; 37:W600–W605.20. Tone M, Powell MJ, Tone Y, Thompson SA, Waldmann H. IL-10 gene expression is controlled by the transcription factors Sp1 and Sp3. J Immunol. 2000; 165:286–291.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Impact of Polymorphisms in the CASPASE Genes on Survival of Patients with Colorectal Cancer
- Association of Dietary Vitamin D and Calcium With Genetic Polymorphisms in Colorectal Neoplasia
- The Colorectal Cancer Risk of Meat Intake, Smoking, and CYP2E1 Polymorphisms: The Comparison of Colorectal Cancer Patients with Controls
- No Association of Insulin-like Growth Factor Gene Polymorphisms with Survival in Patients with Colorectal Cancer
- In silico Identification of SFRP1 as a Hypermethylated Gene in Colorectal Cancers