J Korean Med Sci.
2010 Apr;25(4):577-582. 10.3346/jkms.2010.25.4.577.
Bioelectrical Impedance May Predict Cell Viability During Ischemia and Reperfusion in Rat Liver
- Affiliations
-
- 1Research Institute of Biomedical Engineering, College of Medicine, Yeungnam University, Daegu, Korea.
- 2Department of Physiology, College of Medicine, Yeungnam University, Daegu, Korea.
- 3Department of Surgery, College of Medicine, Yeungnam University, Daegu, Korea.
- KMID: 1792928
- DOI: http://doi.org/10.3346/jkms.2010.25.4.577
Abstract
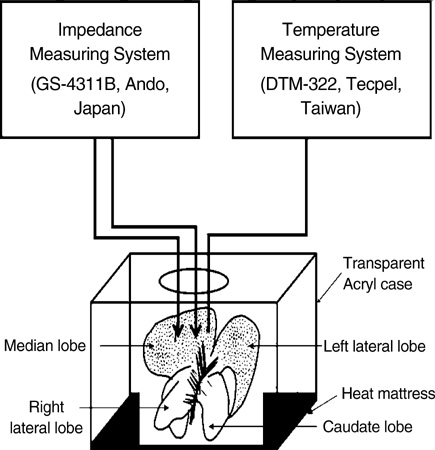
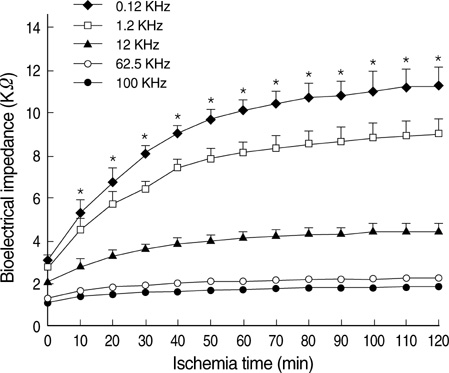
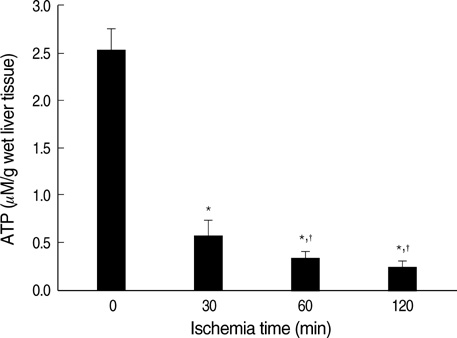
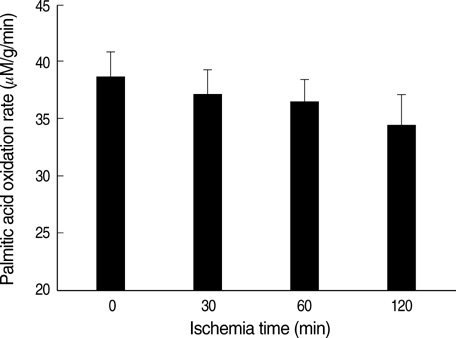
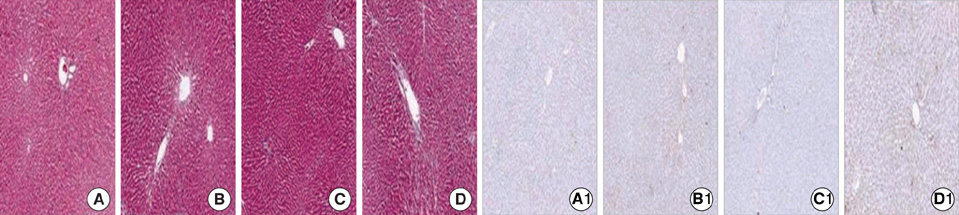
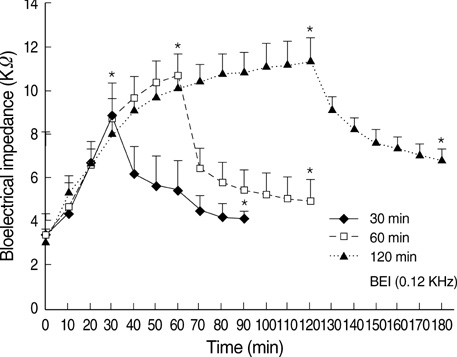
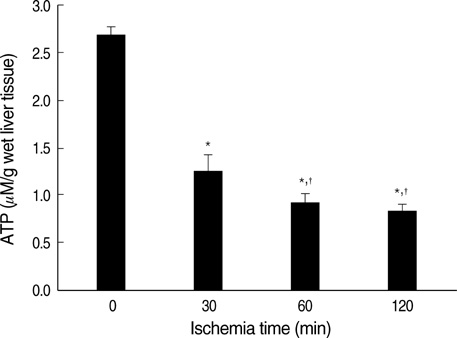
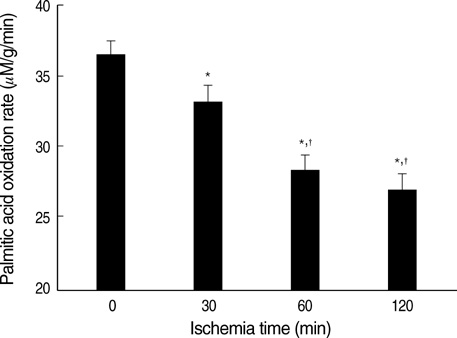
- Ischemia and reperfusion (I/R) injury is a major cause of hepatic failure after liver surgery, but no method could monitor or predict it real-time during surgery. We measured bioelectrical impedance (BEI) and cell viability to assess the usefulness of BEI during I/R in rat liver. A 70% partial liver ischemia model was used. BEI was measured at various frequencies. Adenosine triphosphate (ATP) content, and palmitic acid oxidation rate were measured, and histological changes were observed in order to quantify liver cell viability. BEI changed significantly during ischemia at low frequency. In the ischemia group, BEI increased gradually during 60 min of ischemia and had a tendency to plateau thereafter. The ATP content decreased below 20% of the baseline level. In the I/R group, BEI recovered to near baseline level. After 24 hr of reperfusion, the ATP contents decreased to below 50% in 30, 60 and 120 min of ischemia and the palmitic acid metabolic rates decreased to 91%, 78%, and 74%, respectively, compared with normal liver. BEI may be a good tool for monitoring I/R during liver surgery. The liver is relatively tolerant to ischemia, however after reperfusion, liver cells may be damaged depending upon the duration of ischemia.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Significance of Bioelectrical Impedance Change after Ischemia and Reperfusion Injury in Liver and What it Causes?
Sung Su Yun
Hanyang Med Rev. 2013;33(3):154-159. doi: 10.7599/hmr.2013.33.3.154.
Reference
-
1. Limdi JK, Hyde GM. Evaluation of abnormal liver function tests. Postgrad Med J. 2003. 79:307–312.
Article2. von Schonfeld J, Erhard J, Beste M, Mahl M, Zotz RB, Lange R, Breuer N, Goebell H, Eigler FW. Conventional and quantitative liver function tests after hepatic transplantation: a prospective long-term follow-up. Transpl Int. 1997. 10:212–216.3. Costa M, Shute B, Mergner WJ. Measurement of ATP synthesis and flocculent matrix densities in mitochondria as a function of 'in vitro' ischemia in the heart and liver of rats. Pathobiology. 1990. 58:129–137.4. Mori K, Ozawa K, Yamamoto Y, Maki A, Shimahara Y, Kobayashi N, Yamaoka Y, Kumada K. Response of hepatic mitochondrial redox state to oral glucose load. Redox tolerance test as a new predictor of surgical risk in hepatectomy. Ann Surg. 1990. 211:438–446.5. Kun S, Ristic B, Peura RA, Dunn RM. Algorithm for tissue ischemia estimation based on electrical impedance spectroscopy. IEEE Trans Biomed Eng. 2003. 50:1352–1359.
Article6. Camargo CA Jr, Madden JF, Gao W, Selvan RS, Clavien PA. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology. 1997. 26:1513–1520.
Article7. Khan HA. Bioluminometric assay of ATP in mouse brain: determinant factors for enhanced test sensitivity. J Biosci. 2003. 28:379–382.
Article8. Kim JY, Koves TR, Yu GS, Gulick T, Cortright RN, Dohm GL, Muoio DM. Evidence of a malonyl-CoA-insensitive carnitine palmitoyltransferase I activity in red skeletal muscle. Am J Physiol Endocrinol Metab. 2002. 282:E1014–E1022.
Article9. Sarin SK, Kumar M. Measuring hepatic functional reserve using MEGX: still a mirage! Indian J Gastroenterol. 2007. 26:203–206.10. Tsubono T, Tsukada K, Hatakeyama K. Hepatic functional reserve in patients with obstructive jaundice: an assessment by the redox tolerance test. Am J Surg. 1995. 169:300–303.
Article11. Jochum C, Beste M, Penndorf V, Farahani MS, Testa G, Nadalin S, Malago M, Broelsch CE, Gerken G. Quantitative liver function tests in donors and recipients of living donor liver transplantation. Liver Transpl. 2006. 12:544–549.
Article12. Zoedler T, Ebener C, Becker H, Roeher HD. Evaluation of liver function tests to predict operative risk in liver surgery. HPB Surg. 1995. 9:13–18.
Article13. Konishi Y, Morimoto T, Kinouchi Y, Iritani T, Monden Y. Electrical properties of extracted rat liver tissue. Res Exp Med (Berl). 1995. 195:183–192.
Article14. Rees AE, Ward LC, Cornish BH, Thomas BJ. Sensitivity of multiple frequency bioelectrical impedance analysis to changes in ion status. Physiol Meas. 1999. 20:349–362.
Article15. Gabriel C, Gabriel S, Corthout E. The dielectric properties of biological tissues: I. Literature survey. Phys Med Biol. 1996. 41:2231–2249.
Article16. Haemmerich D, Ozkan R, Tungjitkusolmun S, Tsai JZ, Mahvi DM, Staelin ST, Webster JG. Changes in electrical resistivity of swine liver after occlusion and postmortem. Med Biol Eng Comput. 2002. 40:29–33.
Article17. Ramalho FS, Fernandez-Monteiro I, Rosello-Catafau J, Peralta C. Hepatic microcirculatory failure. Acta Cir Bras. 2006. 21:Suppl 1. 48–53.
Article18. Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988. 45:673–676.
Article19. Kim SK, Jee D, Kim JY, Choi JH. Effects of propofol on early phase of warm hepatic ischemia/reperfusion injury. Hepatogastroenterology. 2007. 54:2333–2336.20. Jeon BR, Yeom DH, Lee SM. Protective effect of allopurinol on hepatic energy metabolism in ischemic and reperfused rat liver. Shock. 2001. 15:112–117.
Article21. Saris NE, Eriksson KO. Mitochondrial dysfunction in ischaemia-reperfusion. Acta Anaesthesiol Scand Suppl. 1995. 107:171–176.
Article22. Huguet C, Gavelli A, Bona S. Hepatic resection with ischemia of the liver exceeding one hour. J Am Coll Surg. 1994. 178:454–458.23. Hannoun L, Borie D, Delva E, Jones D, Vaillant JC, Nordlinger B, Parc R. Liver resection with normothermic ischaemia exceeding 1 h. Br J Surg. 1993. 80:1161–1165.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Significance of Bioelectrical Impedance during Ischemia-Reperfusion Injury in the Rabbit's Liver
- Significance of Bioelectrical Impedance Change after Ischemia and Reperfusion Injury in Liver and What it Causes?
- Estimation of Liver Cell Viability after Ischemia and Reperfusion Injury in Rat Liver
- Impaired Cation Transport May Lead to Bioelectrical Impedance Changes during Hepatic Ischemia
- Changes in HO-1, HSP70 and iNOS Expressions in the Rat Liver after Remote Ischemic Preconditioning