J Korean Med Sci.
2010 Apr;25(4):564-569. 10.3346/jkms.2010.25.4.564.
Influence of Transforming Growth Factor-beta1 Gene Polymorphism at Codon 10 on the Development of Cirrhosis in Chronic Hepatitis B Virus Carriers
- Affiliations
-
- 1Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea. osshsjuj@yahoo.co.kr
- KMID: 1792926
- DOI: http://doi.org/10.3346/jkms.2010.25.4.564
Abstract
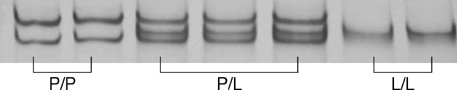
- Transforming growth factor (TGF)-beta1 is a key cytokine producing extracellular matrix. We evaluated the effect of TGF-beta1 gene polymorphism at codon 10 on the development of cirrhosis in patients with chronic hepatitis B. One hundred seventy eight patients with chronic hepatitis (CH, n=57) or liver cirrhosis (LC, n=121), who had HBsAg and were over 50 yr old, were enrolled. The genotypes were determined by single strand conformation polymorphism. There were no significant differences in age and sex ratio between CH and LC groups. HBeAg positivity and detection rate of HBV DNA were higher in LC than in CH groups (P=0.055 and P=0.003, respectively). There were three types of TGF-beta1 gene polymorphism at codon 10: proline homozygous (P/P), proline/leucine heterozygous (P/L), and leucine homozygous (L/L) genotype. In CH group, the proportions of P/P, P/L, and L/L genotype were 32%, 51%, and 17%, respectively. In LC group, the proportions of those genotypes were 20%, 47%, and 33%, respectively. The L/L genotype was presented more frequently in LC than in CH groups (P=0.017). Multivariate logistic regression analysis confirms that detectable HBV DNA (odds ratio [OR]: 3.037, 95% confidence interval [CI]: 1.504-6.133, P=0.002) and L/L genotype (OR: 3.408, 95% CI: 1.279-9.085, P=0.014) are risk factors for cirrhosis.
MeSH Terms
-
Aged
Asian Continental Ancestry Group/genetics
*Carrier State
*Codon
Female
Genetic Predisposition to Disease
Genotype
Hepatitis B virus/genetics
*Hepatitis B, Chronic/genetics/virology
Humans
*Liver Cirrhosis/genetics/virology
Male
Middle Aged
Odds Ratio
*Polymorphism, Genetic
Risk Factors
Transforming Growth Factor beta1/*genetics
Codon
Transforming Growth Factor beta1
Figure
Reference
-
1. Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000. 342:1350–1358.2. Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis. 2001. 21:397–416.
Article3. Cambien F, Ricard S, Troesch A, Mallet C, Generenaz L, Evans A, Arveiler D, Luc G, Ruidavets JB, Poirier O. Polymorphisms of the transforming growth factor-beta 1 gene in relation to myocardial infarction and blood pressure. The Etude Cas-Témoin de l'Infarctus du Myocarde (ECTIM) Study. Hypertension. 1996. 28:881–887.4. Awad MR, El-Gamel A, Hasleton P, Turner DM, Sinnott PJ, Hutchinson IV. Genotypic variation in the transforming growth factor-beta1 gene: association with transforming growth factor-beta1 production, fibrotic lung disease, and graft fibrosis after lung transplantation. Transplantation. 1998. 66:1014–1020.5. Powell EE, Edwards-Smith CJ, Hay JL, Clouston AD, Crawford DH, Shorthouse C, Purdie DM, Jonsson JR. Host genetic factors influence disease progression in chronic hepatitis C. Hepatology. 2000. 31:828–833.
Article6. Suzuki S, Tanaka Y, Orito E, Sugauchi F, Hasegawa I, Sakurai M, Fujiwara K, Ohno T, Ueda R, Mizokami M. Transforming growth factor-beta-1 genetic polymorphism in Japanese patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2003. 18:1139–1143.
Article7. Migita K, Miyazoe S, Maeda Y, Daikoku M, Abiru S, Ueki T, Yano K, Nagaoka S, Matsumoto T, Nakao K, Hamasaki K, Yatsuhashi H, Ishibashi H, Eguchi K. Cytokine gene polymorphisms in Japanese patients with hepatitis B virus infection--association between TGF-beta1 polymorphisms and hepatocellular carcinoma. J Hepatol. 2005. 42:505–510.8. Gewaltig J, Mangasser-Stephan K, Gartung C, Biesterfeld S, Gressner AM. Association of polymorphisms of the transforming growth factor-beta1 gene with the rate of progression of HCV-induced liver fibrosis. Clin Chim Acta. 2002. 316:83–94.9. Wang H, Mengsteab S, Tag CG, Gao CF, Hellerbrand C, Lammert F, Gressner AM, Weiskirchen R. Transforming growth factor-beta1 gene polymorphisms are associated with progression of liver fibrosis in Caucasians with chronic hepatitis C infection. World J Gastroenterol. 2005. 11:1929–1936.10. Kim YJ, Lee HS, Im JP, Min BH, Kim HD, Jeong JB, Yoon JH, Kim CY, Kim MS, Kim JY, Jung JH, Kim LH, Park BL, Shin HD. Association of transforming growth factor-beta1 gene polymorphisms with a hepatocellular carcinoma risk in patients with chronic hepatitis B virus infection. Exp Mol Med. 2003. 35:196–202.11. Yokota M, Ichihara S, Lin TL, Nakashima N, Yamada Y. Association of a T29-->C polymorphism of the transforming growth factor-beta1 gene with genetic susceptibility to myocardial infarction in Japanese. Circulation. 2000. 101:2783–2787.12. Kwon OS, Jang US, Chang CS, Song KS, Yu SK, Kwon KA, Park DK, Kim YS, Kim YS, Choi DJ, Kim JH. Relation of genetic polymorphism at codon 10 in transfroming growth factor-beta1 gene with occurence of liver cirrhosis and progression of hepatocellular carcinoma with HBsAg. Gut. 2006. 55:26A.13. Kang HG, Chae MH, Park JM, Kim EJ, Park JH, Kam S, Cha SI, Kim CH, Park RW, Park SH, Kim YL, Kim IS, Jung TH, Park JY. Polymorphisms in TGF-beta1 gene and the risk of lung cancer. Lung Cancer. 2006. 52:1–7.14. Kim SJ, Park K, Koeller D, Kim KY, Wakefield LM, Sporn MB, Roberts AB. Post-transcriptional regulation of the human transforming growth factor-beta 1 gene. J Biol Chem. 1992. 267:13702–13707.
Article15. Gressner AM, Dooley S, Weiskirchen R. Dufour JF, Clavien PA, editors. TGF-β and the smad pathway in liver fibrogenesis. Signaling Pathways in Liver Diseases. 2005. Germany: Springer;139–150.
Article16. Bataller R, North KE, Brenner DA. Genetic polymorphisms and the progression of liver fibrosis: a critical appraisal. Hepatology. 2003. 37:493–503.
Article17. Verner K, Schatz G. Protein translocation across membranes. Science. 1988. 241:1307–1313.
Article18. Randall LL, Hardy SJ. Unity in function in the absence of consensus in sequence: role of leader peptides in export. Science. 1989. 243:1156–1159.
Article19. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007. 132:2557–2576.
Article20. Kim CY, Kim JW, Lee HS, Yoon YB, Song IS. Natural history and survival rate of chronic liver disease in Korea. Korean J Med. 1994. 46:168–180.21. Lok AS, McMahon BJ. Chronic hepatitis B: update of recommendations. Hepatology. 2004. 39:857–861.
Article22. Lok AS, Lai CL, Wu PC, Leung EK, Lam TS. Spontaneous hepatitis B e antigen to antibody seroconversion and reversion in Chinese patients with chronic hepatitis B virus infection. Gastroenterology. 1987. 92:1839–1843.
Article23. Yuen MF, Lai CL. Natural history of chronic hepatitis B virus infection. J Gastroenterol Hepatol. 2000. 15:Suppl. E20–E24.
Article24. Hui CK, Leung N, Shek TW, Yao H, Lee WK, Lai JY, Lai ST, Wong WM, Lai LS, Poon RT, Lo CM, Fan ST, Lau GK. Sustained disease remission after spontaneous HBeAg seroconversion is associated with reduction in fibrosis progression in chronic hepatitis B Chinese patients. Hepatology. 2007. 46:690–698.
Article25. Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenerology. 2006. 130:678–686.
Article26. D'Amico G, Pagliaro L, Bosch J. Pharmacological treatment of portal hypertension: an evidence-based approach. Semin Liver Dis. 1999. 19:475–505.27. Karasu Z, Tekin F, Ersoz G, Gunsar F, Batur Y, Ilter T, Akarca US. Liver fibrosis is associated with decreased peripheral platelet count in patients with chronic hepatitis B and C. Dig Dis Sci. 2007. 52:1535–1539.
Article28. Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007. 46:912–921.
Article29. Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J, Cross A, DeHertogh D, Wilber R, Colonno R, Apelian D. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006. 354:1001–1010.
Article30. Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004. 351:1521–1531.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Genetic polymorphism at codon 10 of the transforming growth factor-beta1 gene in patients with alcoholic liver cirrhosis
- Transforming Growth Factor-beta 1 Gene Polymorphisms According to Diabetic Nephropathy in Type 2 Diabetes
- Polymorphism in Codons 10 and 25 of the Transforming Growth Factor-beta1 Gene in Korean Population and in Patients with Liver Cirrhosis and Hepatocellular Carcinoma
- Missense Mutationsof Hepatitis B Virus Core Gene in Chronic Hepatitis B Virus Carriers
- Association of Polymorphisms of the TNF-alpha and TGF-beta1 Genes with Renal Allograft Dysfunction