Korean J Gastroenterol.
2012 Jun;59(6):395-400. 10.4166/kjg.2012.59.6.395.
Pancreatic Cancer and MicroRNAs
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. woltoong@snu.ac.kr
- KMID: 1792843
- DOI: http://doi.org/10.4166/kjg.2012.59.6.395
Abstract
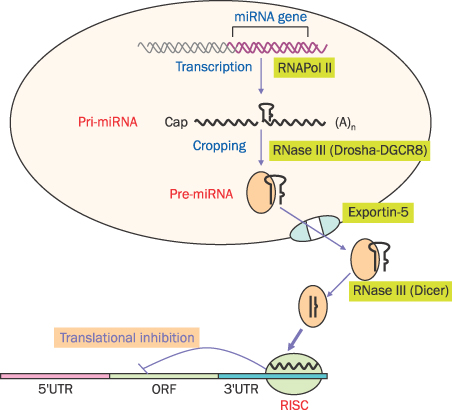
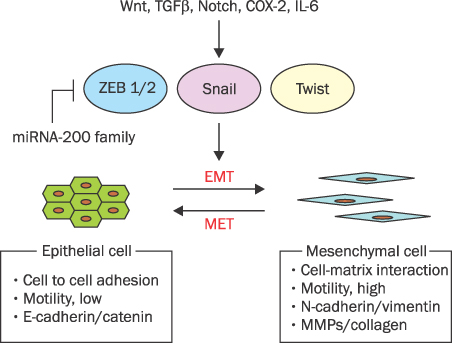
- MicroRNAs (miRNAs) are recently discovered non-coding small RNAs that play a role as regulators of genetic expressions in eukaryotic cells. It comprises about 20 nucleotides, which contains seed sequence to bind 3'-untranslated lesion of specific target mRNA. It regulates self-renewal, proliferation and differentiation via post-transcriptional gene slicing in normal situation. Aberrant expressions of miRNAs are observed in many cancers as well. miRNAs in cancer cells have been investigated extensively to have a role in tumorigenesis, invasion, metastasis and chemoresistance. In cancer cells, miRNAs act both as tumor suppressors or oncogenes by doing down-regulation of oncogenes or up-regulation of tumor suppressors, respectively. This suggests miRNAs can be potential therapeutic and diagnostic targets in cancers. Pancreatic cancer is one of the most lethal tumors. In spite of many efforts, overall 5-year survival rate of pancreatic cancer is still very low (<5%). Recently, several miRNAs as an oncomir (acting like oncogenes or tumor suppressor genes) are discovered in pancreatic cancer. Here, the role of miRNAs in pancreatic cancer will be discussed and its possibility of diagnostic/therapeutic target will be also mentioned.
MeSH Terms
Figure
Reference
-
1. Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993. 75:843–854.2. Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2002. 99:15524–15529.3. Soto-Reyes E, González-Barrios R, Cisneros-Soberanis F, et al. Disruption of CTCF at the miR-125b1 locus in gynecological cancers. BMC Cancer. 2012. 12:40.4. Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005. 435:834–838.5. Farazi TA, Spitzer JI, Morozov P, Tuschl T. miRNAs in human cancer. J Pathol. 2011. 223:102–115.6. Rinaldi A, Poretti G, Kwee I, et al. Concomitant MYC and microRNA cluster miR-17-92 (C13orf25) amplification in human mantle cell lymphoma. Leuk Lymphoma. 2007. 48:410–412.7. Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007. 133:647–658.8. Lu Z, Liu M, Stribinskis V, et al. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 2008. 27:4373–4379.9. Johnson SM, Grosshans H, Shingara J, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005. 120:635–647.10. Tarasov V, Jung P, Verdoodt B, et al. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle. 2007. 6:1586–1593.11. Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007. 449:682–688.12. Huang Q, Gumireddy K, Schrier M, et al. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol. 2008. 10:202–210.13. Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008. 10:593–601.14. Burk U, Schubert J, Wellner U, et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008. 9:582–589.15. Tavazoie SF, Alarcón C, Oskarsson T, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008. 451:147–152.16. Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997. 3:730–737.17. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003. 100:3983–3988.18. Bloomston M, Frankel WL, Petrocca F, et al. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007. 297:1901–1908.19. Szafranska AE, Doleshal M, Edmunds HS, et al. Analysis of microRNAs in pancreatic fine-needle aspirates can classify benign and malignant tissues. Clin Chem. 2008. 54:1716–1724.20. Habbe N, Koorstra JB, Mendell JT, et al. MicroRNA miR-155 is a biomarker of early pancreatic neoplasia. Cancer Biol Ther. 2009. 8:340–346.21. Ho AS, Huang X, Cao H, et al. Circulating miR-210 as a novel hypoxia marker in pancreatic cancer. Transl Oncol. 2010. 3:109–113.22. Kong X, Du Y, Wang G, et al. Detection of differentially expressed microRNAs in serum of pancreatic ductal adenocarcinoma patients: miR-196a could be a potential marker for poor prognosis. Dig Dis Sci. 2011. 56:602–609.23. Ali S, Ahmad A, Banerjee S, et al. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010. 70:3606–3617.24. Hwang JH, Voortman J, Giovannetti E, et al. Identification of microRNA-21 as a biomarker for chemoresistance and clinical outcome following adjuvant therapy in resectable pancreatic cancer. PLoS One. 2010. 5:e10630.25. Li Y, VandenBoom TG 2nd, Kong D, et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009. 69:6704–6712.26. Weiss FU, Marques IJ, Woltering JM, et al. Retinoic acid receptor antagonists inhibit miR-10a expression and block metastatic behavior of pancreatic cancer. Gastroenterology. 2009. 137:2136–2145.27. Ji Q, Hao X, Zhang M, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One. 2009. 4:e6816.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Surveillance and Early Diagnosis of Pancreatic Cancer
- MicroRNA expression profiling of diagnostic needle aspirates from surgical pancreatic cancer specimens
- α, γ-Mangostins Induce Autophagy and Show Synergistic Effect with Gemcitabine in Pancreatic Cancer Cell Lines
- The Clinical Utility of MicroRNA as a Prognostic Biomarker of Pancreatobiliary Cancers
- MicroRNA Expression Pattern in Intraductal Papillary Mucinous Neoplasm