Lab Anim Res.
2015 Mar;31(1):33-39. 10.5625/lar.2015.31.1.33.
The relaxing effect of Poncirus fructus and its flavonoid content on porcine coronary artery
- Affiliations
-
- 1Department of Physiology, College of Veterinary Medicine, Kyungpook National University, Daegu, Korea. twkim@mail.knu.ac.kr
- 2Metropolitan Health & Environmental Research Institute, Daegu, Korea.
- 3Department of Oral Physiology, School of Dentistry, Kyungpook National University, Daegu, Korea.
- KMID: 1787649
- DOI: http://doi.org/10.5625/lar.2015.31.1.33
Abstract
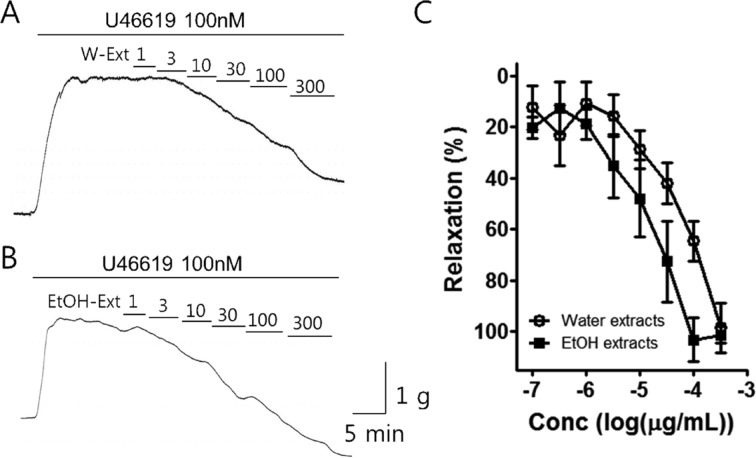
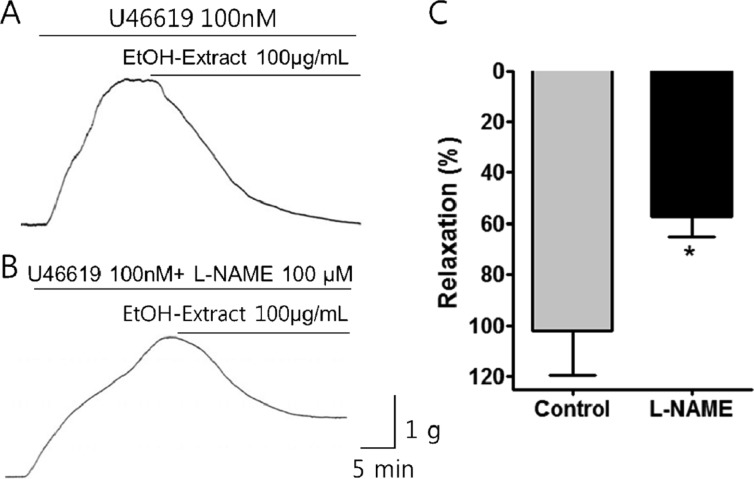
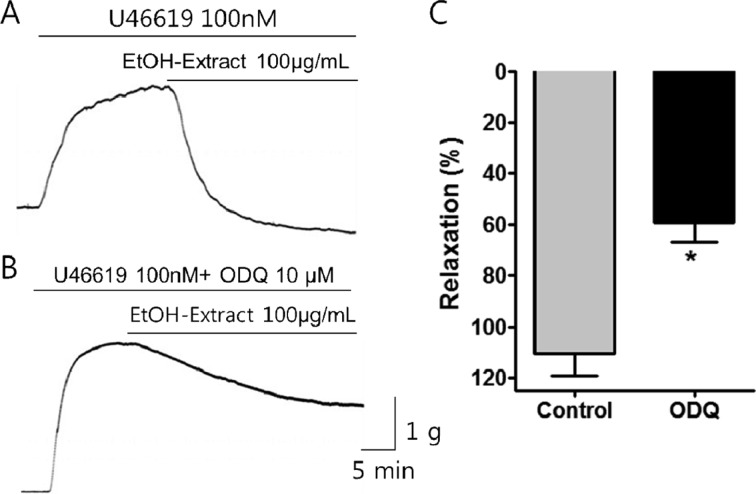
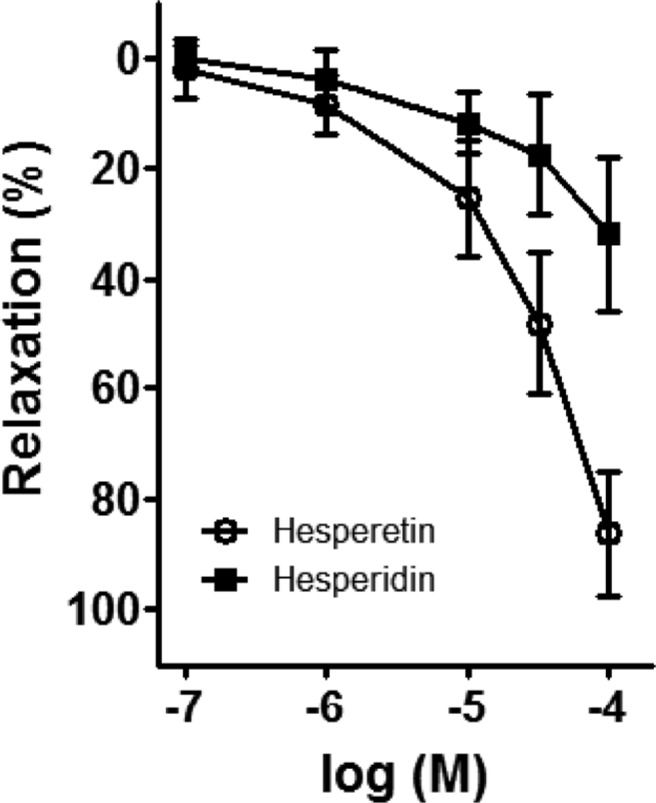
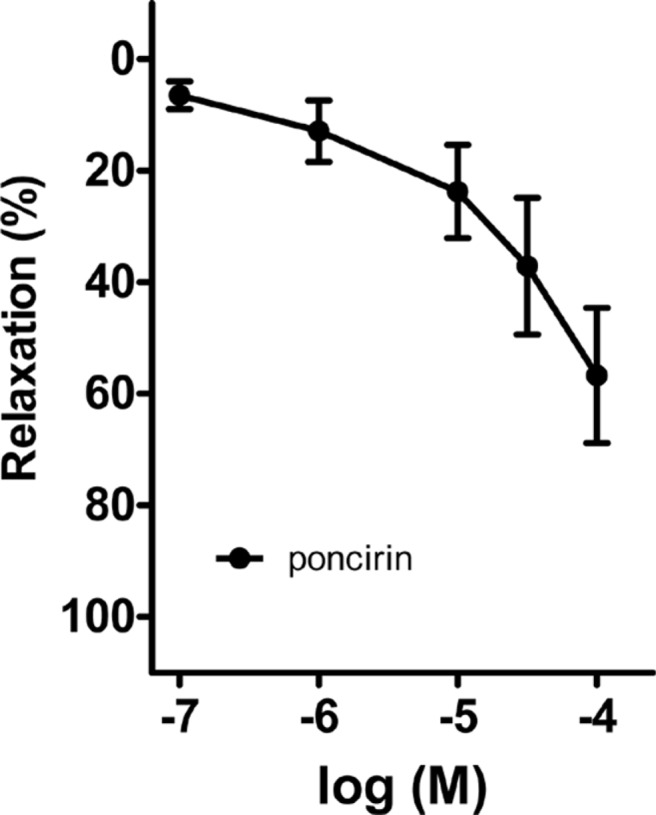
- Coronary artery disease is a common occurrence in human, and causes enormous social cost. Poncirus fructus (PF), the dried immature fruits of Poncirus trifoliata Rafinesquem, is used in the treatment of womb contraction and dyspepsia, as a prokinetic, and in improving blood circulation. This study was performed to investigate the effects of PF and some of its flavonoids components on the coronary from the pig. The arterial ring was suspended by a pair of stainless steel stirrups in an organ bath. The end of the upper stirrup was connected to an isometric force transducer. A dose-dependent induction of relaxation was observed by both water and 70% ethanol extracts of PF in the porcine coronary artery precontracted with U46619 (100 nM), a stable analogue of the potent vasoconstrictor thromboxane A2. The 70% ethanol extract showed more efficacy than the water extract. Pretreatment of the artery with L-NAME (100 microM), a nitric oxide synthase inhibitor, resulted in a significant reduction in the relaxation induced by PF extract. In addition, ODQ (10 microM), a soluble guanylate cyclase inhibitor, also significantly reduced the effects of PF extracts. Hesperidin, a flavonoid present in PF, induced very weak relaxation of the porcine coronary artery at a high concentration (100 microM), while its aglycone, hesperetin, demonstrated a dose-dependent relaxation. In conclusion, PF extracts induced relaxation in the porcine coronary artery, partially through the nitric oxide-cGMP pathway, and the aglycones of flavonoids might be also involved in the relaxation of the same artery.
MeSH Terms
-
15-Hydroxy-11 alpha,9 alpha-(epoxymethano)prosta-5,13-dienoic Acid
Arteries
Baths
Blood Circulation
Coronary Artery Disease
Coronary Vessels*
Dyspepsia
Ethanol
Flavonoids
Fruit
Guanylate Cyclase
Hesperidin
Humans
NG-Nitroarginine Methyl Ester
Nitric Oxide Synthase
Poncirus*
Relaxation
Stainless Steel
Thromboxane A2
Transducers
Water
15-Hydroxy-11 alpha,9 alpha-(epoxymethano)prosta-5,13-dienoic Acid
Ethanol
Flavonoids
Guanylate Cyclase
Hesperidin
NG-Nitroarginine Methyl Ester
Nitric Oxide Synthase
Stainless Steel
Thromboxane A2
Water
Figure
Reference
-
1. Jones DW, Chambless LE, Folsom AR, Heiss G, Hutchinson RG, Sharrett AR, Szklo M, Taylor HA Jr. Risk factors for coronary heart disease in African Americans: the atherosclerosis risk in communities study, 1987-1997. Arch Intern Med. 2002; 162(22):2565–2571. PMID: 12456228.2. Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009; 119(3):480–486. PMID: 19171871.3. KAPE. Handbook of the Korea Pharmacopoeia. Seoul: Shinilbooks;2008.4. Park KC, Bae GS, Choi SB, Jo IJ, Gwak TS, S. LG, Park SJ, Song HJ. Protective effect of Poncirus trifoliata and Citrus aurantium extract on acute pancreatitis in mice model. Korean J Herbol. 2012; 27(5):9–14.
Article5. Lee YM, Kim DK, Kim SH, Shin TY, Kim HM. Antianaphylactic activity of Poncirus trifoliata fruit extract. J Ethnopharmacol. 1996; 54(2-3):77–84. PMID: 8953421.
Article6. Ban SS, Yoon HD, Shin OC, Shin YJ, Park CS, Park JH, Seo BI. The Effects of Artemisiae Capillaris, Ponciri Fructus and Cartaegi Fructus in Obese Rats Induced by High Fat Diet. Korean J Herbol. 2006; 21(3):55–67.7. Ham IH, Lee UC, Lee BH, Choi HY. Lipid Lowering activity of Ponciri Fructus and Aurantii Fructus Immaturus on hyperlipemia rats induced by Triton WR-1339. Korean J Herbol. 2007; 22(3):109–116.8. Son AR, Choi JY, Kim JA, Cho SH, Xu GH, Park SH, Chung SR, Chung TC, Jahng YD, Son JK, Lee SH. Isolation of Melanogenesis Inhibitors from Ponciri Fructus. Korean J Pharmacogn. 2005; 36(1):1–8.9. Shim WS, Back H, Seo EK, Lee HT, Shim CK. Long-term administration of an aqueous extract of dried, immature fruit of Poncirus trifoliata (L.) Raf. suppresses body weight gain in rats. J Ethnopharmacol. 2009; 126(2):294–299. PMID: 19703543.
Article10. Lim JH, Kim HS, Choi EJ, Shim CK, Park HJ. Effects of Poncirus Fructus on gastrointestinal motility in guinea pig. Korean J Neurogastroenterol Motil. 2008; 14(1):7–17.11. Lee HT, Seo EK, Chung SJ, Shim CK. Prokinetic activity of an aqueous extract from dried immature fruit of Poncirus trifoliata (L.) Raf. J Ethnopharmacol. 2005; 102(2):131–136. PMID: 16191468.
Article12. Choi KH, Jeong SI, Hwang BS, Lee JH, Ryoo HK, Lee S, Choi BK, Jung KY. Hexane extract of Poncirus trifoliata (L.) Raf. stimulates the motility of rat distal colon. J Ethnopharmacol. 2010; 127(3):718–724. PMID: 19963058.
Article13. Kim TW. Effects of Poncirus Fructus and Aurantii Fructus Immaturus on the gastric Fundus Motility. J Vet Clin. 2013; 30(1):1–4.14. Kim CM, Shin MK, Ahn DG, Lee KS. Chungyak Daesajun. Seoul: Jungdam Publisher;1997.15. Ajay M, Gilani AU, Mustafa MR. Effects of flavonoids on vascular smooth muscle of the isolated rat thoracic aorta. Life Sci. 2003; 74(5):603–612. PMID: 14623031.
Article16. Chan EC, Pannangpetch P, Woodman OL. Relaxation to flavones and flavonols in rat isolated thoracic aorta: mechanism of action and structure-activity relationships. J Cardiovasc Pharmacol. 2000; 35(2):326–333. PMID: 10672869.
Article17. Fan YF, Chen ZW, Guo Y, Wang QH, Song B. Cellular mechanisms underlying Hyperin-induced relaxation of rat basilar artery. Fitoterapia. 2011; 82(4):626–631. PMID: 21300141.
Article18. Flesch M, Schwarz A, Böhm M. Effects of red and white wine on endothelium-dependent vasorelaxation of rat aorta and human coronary arteries. Am J Physiol. 1998; 275(4 Pt 2):H1183–H1190. PMID: 9746465.
Article19. Pérez-Vizcaíno F, Ibarra M, Cogolludo AL, Duarte J, Zaragozá-Arnáez F, Moreno L, López-López G, Tamargo J. Endotheliumindependent vasodilator effects of the flavonoid quercetin and its methylated metabolites in rat conductance and resistance arteries. J Pharmacol Exp Ther. 2002; 302(1):66–72. PMID: 12065701.
Article20. Roghani M, Baluchnejadmojarad T, Vaez-Mahdavi MR, Roghani-Dehkordi F. Mechanisms underlying quercetin-induced vasorelaxation in aorta of subchronic diabetic rats: an in vitro study. Vascul Pharmacol. 2004; 42(1):31–35. PMID: 15664885.
Article21. KFDA. The Korea Pharmacopoeia. Seoul: KFDA;2008.22. Nugroho A, Park MG, Jin SE, Choi JS, Park HJ. Quantitative Analsysis of Flavanone Glycosides and Peroxynitrite Scavenging Effect of the Five Oriental Medicinal Drugs (Aurantii nobilis Pericarpium, Citrii unshiu Pericarpium, Citrii unshiu Semen, Aurantii Fructus, Poncirii Fructus). Korean J Pharmacogn. 2009; 40(4):370–375.23. Malagò R, Pezzato A, Barbiani C, Alfonsi U, Nicolì L, Caliari G, Pozzi Mucelli R. Coronary artery anatomy and variants. Pediatr Radiol. 2011; 41(12):1505–1515. PMID: 22127682.
Article24. Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005; 111(25):3481–3488. PMID: 15983262.
Article25. Jawad E, Arora R. Chronic stable angina pectoris. Dis Mon. 2008; 54(9):671–689. PMID: 18725007.
Article26. Toufektsian MC, de Lorgeril M, Nagy N, Salen P, Donati MB, Giordano L, Mock HP, Peterek S, Matros A, Petroni K, Pilu R, Rotilio D, Tonelli C, de Leiris J, Boucher F, Martin C. Chronic dietary intake of plant-derived anthocyanins protects the rat heart against ischemia-reperfusion injury. J Nutr. 2008; 138(4):747–752. PMID: 18356330.
Article27. Cassidy A, Rimm EB, O'Reilly EJ, Logroscino G, Kay C, Chiuve SE, Rexrode KM. Dietary flavonoids and risk of stroke in women. Stroke. 2012; 43(4):946–951. PMID: 22363060.
Article28. Mursu J, Voutilainen S, Nurmi T, Tuomainen TP, Kurl S, Salonen JT. Flavonoid intake and the risk of ischaemic stroke and CVD mortality in middle-aged Finnish men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br J Nutr. 2008; 100(4):890–895. PMID: 18377681.
Article29. Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002; 96(2-3):67–202. PMID: 12453566.
Article30. Edwards G, Félétou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch. 2010; 459(6):863–879. PMID: 20383718.
Article31. Feletou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond). 2009; 117(4):139–155. PMID: 19601928.32. Khoo NK, White CR, Pozzo-Miller L, Zhou F, Constance C, Inoue T, Patel RP, Parks DA. Dietary flavonoid quercetin stimulates vasorelaxation in aortic vessels. Free Radic Biol Med. 2010; 49(3):339–347. PMID: 20423726.
Article33. Bokkenheuser VD, Shackleton CH, Winter J. Hydrolysis of dietary flavonoid glycosides by strains of intestinal Bacteroides from humans. Biochem J. 1987; 248(3):953–956. PMID: 3435494.
Article34. Griffiths LA, Barrow A. Metabolism of flavonoid compounds in germ-free rats. Biochem J. 1972; 130(4):1161–1162. PMID: 4656801.
Article35. Walle T, Browning AM, Steed LL, Reed SG, Walle UK. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. J Nutr. 2005; 135(1):48–52. PMID: 15623831.
Article36. Xu YC, Leung SW, Yeung DK, Hu LH, Chen GH, Che CM, Man RY. Structure-activity relationships of flavonoids for vascular relaxation in porcine coronary artery. Phytochemistry. 2007; 68(8):1179–1188. PMID: 17395220.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of Endothelium -Derived Relaxing Factor in the Pathogenesis of Coronary Artery Spasm and Its Relationship with Ethanol
- Effects of Ketamine on the Ca(2+) Channel and K(+) Channel of the Porcine Coronary Artery
- Effects of Poncirus fructus on Gastrointestinal Motility in Guinea Pig : in vitro and in vivo Study
- Ultraviolet Light-Induced Relaxant Response in Arterial Smooth Muscles, Mediators of the Response and Effect of Calcium Modulators on the Relaxation
- A study of the lipoprotein lipase inhibitory mechanism of Poncirus trifoliata water extracts