J Korean Med Sci.
2013 Apr;28(4):593-601. 10.3346/jkms.2013.28.4.593.
Alpha Internexin Expression Related with Molecular Characteristics in Adult Glioblastoma and Oligodendroglioma
- Affiliations
-
- 1Department of Pathology, Seoul National University Hospital, Seoul, Korea. shparknp@snu.ac.kr
- 2Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea.
- KMID: 1786969
- DOI: http://doi.org/10.3346/jkms.2013.28.4.593
Abstract
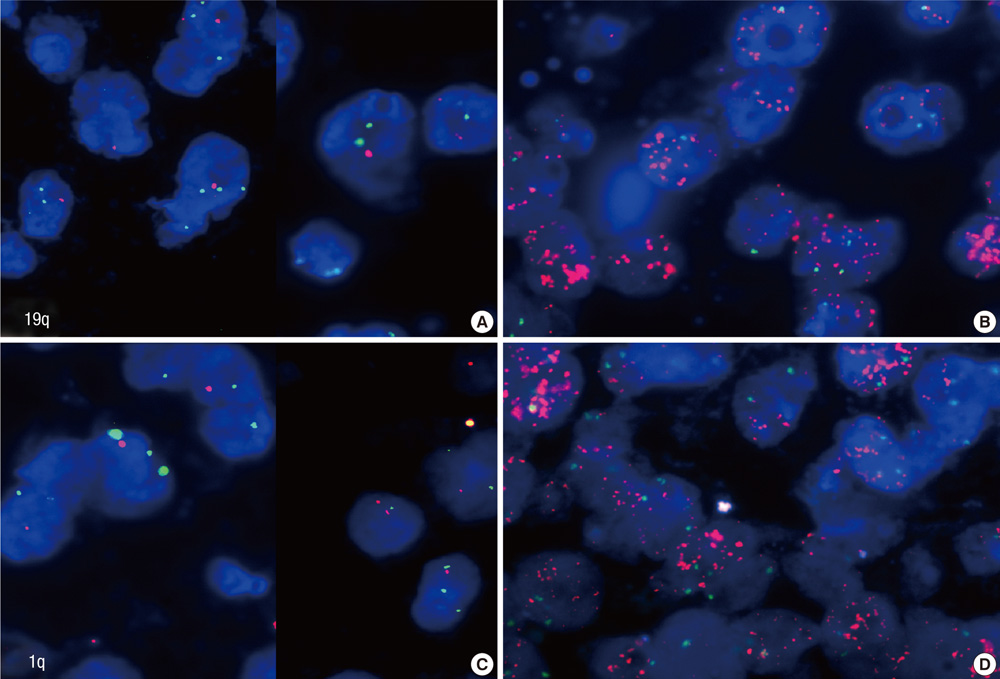
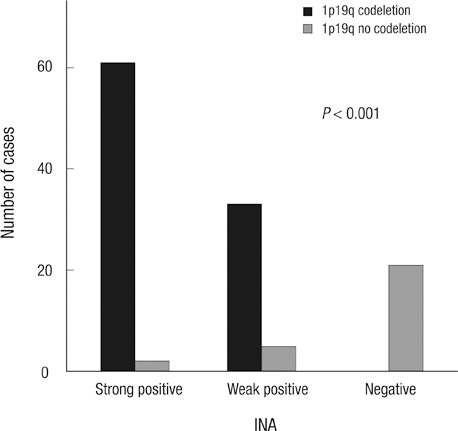
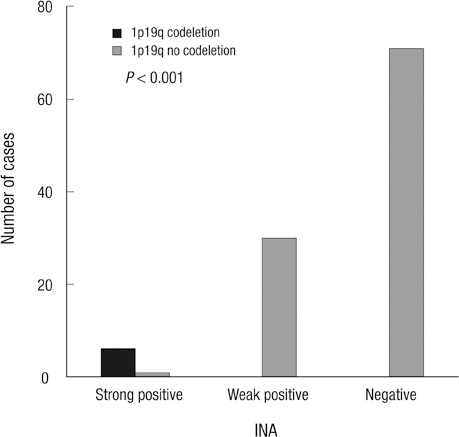
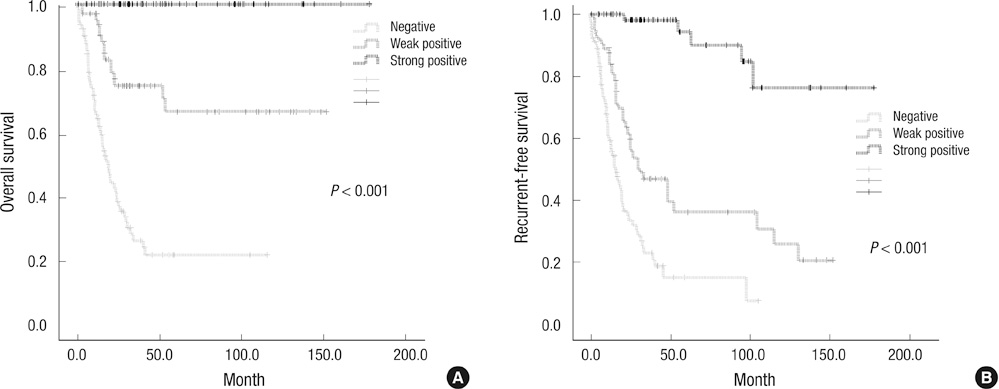
- Alpha-internexin (INA) is a proneuronal gene-encoding neurofilament interacting protein. INA is overexpressed mostly in oligodendroglial phenotype gliomas, is related to 1p/19q codeletion, and is a favorable prognostic marker. We studied INA expression in oligodendrogliomas (ODGs) and glioblastomas (GBMs) to verify its association with several molecular phenotypes, 1p/19q codeletion, and epidermal growth-factor-receptor (EGFR) amplification. A total of 230 low- and high-grade ODG and GBM cases was analyzed for INA expression by immunohistochemical staining; and 1p/19q and EGFR gene status was examined by fluorescence in-situ hybridization. INA was positive in 80.3% of ODGs and in 34.3% of GBMs. 1p/19q codeletion was detected in 77.0% of ODGs and 5.5% of GBMs. INA and 1p/19q codeletion were strongly correlated (P < 0.001). The specificity of INA expression for 1p/19q codeletion was 70.8%, while sensitivity was 100%; positive predictive value was 72.5%, and negative predictive value was 29.2% in all 228 tumors. INA expression was correlated with better progression-free survival (PFS) and overall survival (OS) (P = 0.001). In conclusion, INA expression has high specificity and sensitivity to predict 1p/19q codeletion, and it is well correlated with PFS of both ODGs and GBMs. Therefore, INA expression could be a simple, reliable, and favorable prognostic and surrogate marker for 1p/19q codeletion and long term survival.
Keyword
MeSH Terms
-
Adult
Brain Neoplasms/*metabolism/mortality/pathology
Chromosomes, Human, Pair 1
Chromosomes, Human, Pair 19
Female
Gene Deletion
Glioblastoma/*metabolism/mortality/pathology
Humans
Immunohistochemistry
In Situ Hybridization, Fluorescence
Intermediate Filament Proteins/genetics/*metabolism
Kaplan-Meier Estimate
Male
Middle Aged
Oligodendroglioma/*metabolism/mortality/pathology
Phenotype
Predictive Value of Tests
Prognosis
Receptor, Epidermal Growth Factor/genetics/metabolism
Intermediate Filament Proteins
Receptor, Epidermal Growth Factor
Figure
Reference
-
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005. 55:74–108.2. Smith JS, Perry A, Borell TJ, Lee HK, O'Fallon J, Hosek SM, Kimmel D, Yates A, Burger PC, Scheithauer BW, et al. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol. 2000. 18:636–645.3. Sanson M, Thillet J, Hoang-Xuan K. Molecular changes in gliomas. Curr Opin Oncol. 2004. 16:607–613.4. Smith JS, Alderete B, Minn Y, Borell TJ, Perry A, Mohapatra G, Hosek SM, Kimmel D, O'Fallon J, Yates A, et al. Localization of common deletion regions on 1p and 19q in human gliomas and their association with histological subtype. Oncogene. 1999. 18:4144–4152.5. Reifenberger J, Reifenberger G, Liu L, James CD, Wechsler W, Collins VP. Molecular genetic analysis of oligodendroglial tumors shows preferential allelic deletions on 19q and 1p. Am J Pathol. 1994. 145:1175–1190.6. McDonald JM, See SJ, Tremont IW, Colman H, Gilbert MR, Groves M, Burger PC, Louis DN, Giannini C, Fuller G, et al. The prognostic impact of histology and 1p/19q status in anaplastic oligodendroglial tumors. Cancer. 2005. 104:1468–1477.7. Lariviere RC, Julien JP. Functions of intermediate filaments in neuronal development and disease. J Neurobiol. 2004. 58:131–148.8. Liem RK, Messing A. Dysfunctions of neuronal and glial intermediate filaments in disease. J Clin Invest. 2009. 119:1814–1824.9. Ho CL, Liem RK. Intermediate filaments in the nervous system: implications in cancer. Cancer Metastasis Rev. 1996. 15:483–497.10. Ducray F, Idbaih A, de Reyniès A, Bièche I, Thillet J, Mokhtari K, Lair S, Marie Y, Paris S, Vidaud M, et al. Anaplastic oligodendrogliomas with 1p19q codeletion have a proneural gene expression profile. Mol Cancer. 2008. 7:41.11. Ducray F, Crinière E, Idbaih A, Mokhtari K, Marie Y, Paris S, Navarro S, Laigle-Donadey F, Dehais C, Thillet J, et al. Alpha-internexin expression identifies 1p19q codeleted gliomas. Neurology. 2009. 72:156–161.12. Dacic S, Shuai Y, Yousem S, Ohori P, Nikiforova M. Clinicopathological predictors of EGFR/KRAS mutational status in primary lung adenocarcinomas. Mod Pathol. 2010. 23:159–168.13. Gozé C, Rigau V, Gibert L, Maudelonde T, Duffau H. Lack of complete 1p19q deletion in a consecutive series of 12 WHO grade II gliomas involving the insula: a marker of worse prognosis? J Neurooncol. 2009. 91:1–5.14. Burger PC, Minn AY, Smith JS, Borell TJ, Jedlicka AE, Huntley BK, Goldthwaite PT, Jenkins RB, Feuerstein BG. Losses of chromosomal arms 1p and 19q in the diagnosis of oligodendroglioma: a study of paraffin-embedded sections. Mod Pathol. 2001. 14:842–853.15. Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001. 37:S9–S15.16. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007. 114:97–109.17. Brat DJ, Parisi JE, Kleinschmidt-DeMasters BK, Yachnis AT, Montine TJ, Boyer PJ, Powell SZ, Prayson RA, McLendon RE. Neuropathology Committee, College of American Pathologists. Surgical neuropathology update: a review of changes introduced by the WHO classification of tumours of the central nervous system, 4th edition. Arch Pathol Lab Med. 2008. 132:993–1007.18. Shaw EJ, Haylock B, Husband D, du Plessis D, Sibson DR, Warnke PC, Walker C. Gene expression in oligodendroglial tumors. Cell Oncol (Dordr). 2011. 34:355–367.19. Velasco A, Pallares J, Santacana M, Yeramian A, Dolcet X, Eritja N, Puente S, Sorolla A, Llecha N, Matias-Guiu X. Loss of heterozygosity in endometrial carcinoma. Int J Gynecol Pathol. 2008. 27:305–317.20. Blokx WA, van Dijk MC, Ruiter DJ. Molecular cytogenetics of cutaneous melanocytic lesions - diagnostic, prognostic and therapeutic aspects. Histopathology. 2010. 56:121–132.21. Riemenschneider MJ, Jeuken JW, Wesseling P, Reifenberger G. Molecular diagnostics of gliomas: state of the art. Acta Neuropathol. 2010. 120:567–584.22. Tabatabai G, Stupp R, van den Bent MJ, Hegi ME, Tonn JC, Wick W, Weller M. Molecular diagnostics of gliomas: the clinical perspective. Acta Neuropathol. 2010. 120:585–592.23. Willoughby V, Sonawala A, Werlang-Perurena A, Donner LR. A comparative immunohistochemical analysis of small round cell tumors of childhood: utility of peripherin and alpha-internexin as markers for neuroblastomas. Appl Immunohistochem Mol Morphol. 2008. 16:344–348.24. Kaya B, Mena H, Miettinen M, Rushing EJ. Alpha-internexin expression in medulloblastomas and atypical teratoid-rhabdoid tumors. Clin Neuropathol. 2003. 22:215–221.25. Figarella-Branger D, Bouvier C, Moroch J, Michalak S, Burel-Vandenbos F. Morphological classification of glioblastomas. Neurochirurgie. 2010. 56:459–463.26. Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006. 9:157–173.27. Lopez-Gines C, Gil-Benso R, Ferrer-Luna R, Benito R, Serna E, Gonzalez-Darder J, Quilis V, Monleon D, Celda B, Cerdá-Nicolas M. New pattern of EGFR amplification in glioblastoma and the relationship of gene copy number with gene expression profile. Mod Pathol. 2010. 23:856–865.28. Durand KS, Guillaudeau A, Weinbreck N, DeArmas R, Robert S, Chaunavel A, Pommepuy I, Bourthoumieu S, Caire F, Sturtz FG, et al. 1p19q LOH patterns and expression of p53 and Olig2 in gliomas: relation with histological types and prognosis. Mod Pathol. 2010. 23:619–628.29. Kuhlmann T, Gutenberg A, Schulten HJ, Paulus W, Rohde V, Bruck W. Nogo-a expression in glial CNS tumors: a tool to differentiate between oligodendrogliomas and other gliomas? Am J Surg Pathol. 2008. 32:1444–1453.30. Ikeda H, Yoshimoto T. Immunohistochemical study of anaplastic meningioma with special reference to the phenotypic change of intermediate filament protein. Ann Diagn Pathol. 2003. 7:214–222.31. Rulseh AM, Keller J, Klener J, Sroubek J, Dbalý V, Syrůček M, Tovaryš F, Vymazal J. Long-term survival of patients suffering from glioblastoma multiforme treated with tumor-treating fields. World J Surg Oncol. 2012. 10:220.32. Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger G, et al. Long-term survival with glioblastoma multiforme. Brain. 2007. 130:2596–2606.33. Yoon KS, Lee MC, Kang SS, Kim JH, Jung S, Kim YJ, Lee JH, Ahn KY, Lee JS, Cheon JY. P53 mutation and epidermal growth factor receptor overexpression in Glioblastoma. J Korean Med Sci. 2001. 16:481–488.