J Korean Med Sci.
2013 Apr;28(4):586-592. 10.3346/jkms.2013.28.4.586.
Evaluation of Immunogenicity and Safety of the New Tetanus-Reduced Diphtheria (Td) Vaccines (GC1107) in Healthy Korean Adolescents: A Phase II, Double-Blind, Randomized, Multicenter Clinical Trial
- Affiliations
-
- 1Department of Pediatrics, The Catholic University of Korea, Seoul, Korea. kjhan@catholic.ac.kr
- 2Department of Pediatrics, Yonsei University, Wonju College of Medicine, Wonju, Korea.
- 3Department of Pediatrics, Chonnam National University Hospital, Gwangju, Korea.
- 4Department of Pediatrics, Changwon Fatima Hospital, Changwon, Korea.
- 5Department of Pediatrics, Korea Cancer Center Hospital, Seoul, Korea.
- 6Research Center, Green Cross Corporation, Yongin, Korea.
- KMID: 1786968
- DOI: http://doi.org/10.3346/jkms.2013.28.4.586
Abstract
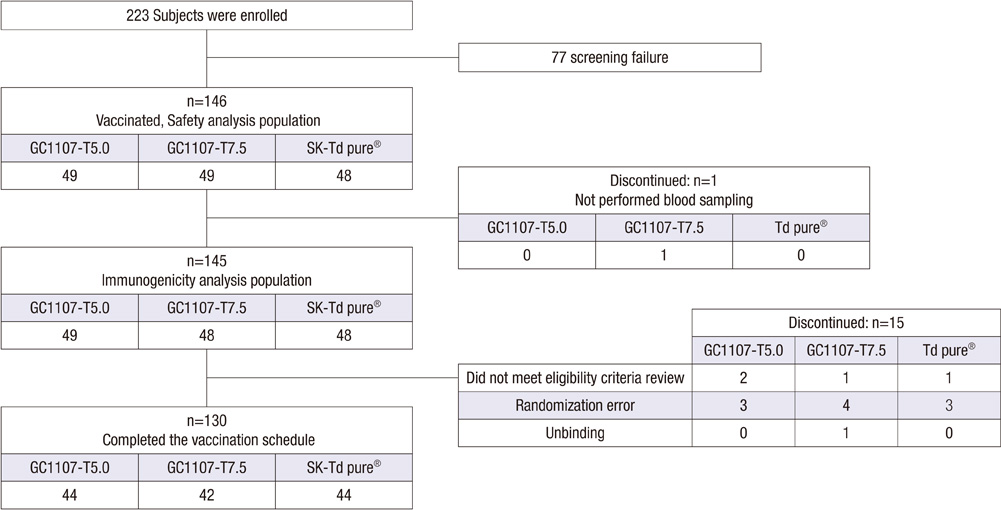
- This phase II clinical trial was conducted to compare the immunogenicity and safety of a newly developed tetanus-reduced diphtheria (Td) vaccine (GC1107-T5.0 and GC1107-T7.5) and control vaccine. This study was also performed to select the proper dose of tetanus toxoid in the new Td vaccines. Healthy adolescents aged between 11 and 12 yr participated in this study. A total of 130 subjects (44 GC1107-T5.0, 42 GC1107-T7.5 and 44 control vaccine) completed a single dose of vaccination. Blood samples were collected from the subjects before and 4 weeks after the vaccination. In this study, all subjects (100%) in both GC1107-T5.0 and GC1107-T7.5 groups showed seroprotective antibody levels (> or = 0.1 U/mL) against diphtheria or tetanus toxoids. After the vaccination, the geometric mean titer (GMT) against diphtheria was significantly higher in Group GC1107-T5.0 (6.53) and GC1107-T7.5 (6.11) than in the control group (3.96). The GMT against tetanus was 18.6 in Group GC1107-T5.0, 19.94 in GC1107-T7.5 and 19.01 in the control group after the vaccination. In this study, the rates of local adverse reactions were 67.3% and 59.1% in GC1107-T5.0 and GC1107-7.5, respectively. No significant differences in the number of adverse reactions, prevalence and degree of severity of the solicited and unsolicited adverse reactions were observed among the three groups. Thus, both newly developed Td vaccines appear to be safe and show good immunogenicity. GC1107-T5.0, which contains relatively small amounts of tetanus toxoid, has been selected for a phase III clinical trial.
MeSH Terms
-
Antibodies, Bacterial/blood
Arthralgia/etiology
Child
Diphtheria/*prevention & control
Diphtheria-Tetanus Vaccine/adverse effects/*immunology
Double-Blind Method
Female
Headache/etiology
Humans
Male
Pain/etiology
Tetanus/*prevention & control
Treatment Outcome
Vaccination
Antibodies, Bacterial
Diphtheria-Tetanus Vaccine
Figure
Reference
-
1. Vitek CR, Wharton M. Plotkin SA, Orenstein WA, Offit PA, editors. Diphtheria toxoid. Vaccines. 2008. 5th ed. Philadelphia: WB Saunders;139–156.2. Wassilak SGF, Roper MH, Kretsinger K, Orenstein WA. Plotkin SA, Orenstein WA, Offit PA, editors. Tetanus toxoid. Vaccines. 2008. 5th ed. Philadelphia: WB Saunders;805–840.3. Kang JH, Hur JK, Kim JH, Lee KI, Park SE, Ma SH, Lee MS, Baek SY, Hong SH, Min HK. Age related seroepidemiological study of diphtheria among Koreans. Korean J Infect Dis. 2000. 32:1–7.4. Kang JH, Hur JK, Kim JH, Lee KI, Park SE, Ma SH, Lee MS, Ban SJ, Hong SH, Cho DH, et al. Age related serosurvey of immunity to tetanus in Korean populations. Korean J Infect Dis. 2001. 33:104–111.5. Lee SY, Kim JS, Ahn JH, Choi JH, Ma SH, Park JS, Kim HM, Kang JH. Immunoassay of diphtheria and tetanus according to ages. Infect Chemother. 2012. 44:62–66.6. Kang JH. The need of Td vaccination according to the changes of tetanus and diphtheria immunity. J Korean Med Assoc. 2008. 51:127–136.7. Lee S, Park WB, Shin KH, Ahn DH, Yoon SH, Cho JY, Shin SG, Jang IJ, Yu KS. Immunogenicity and safety of a single intramuscular dose of a diphtheria-tetanus toxoid (Td) vaccine (GC1107) in Korean adults. Vaccine. 2011. 29:7638–7643.8. Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. 2004. 8th ed. Atlanta: CDC;65–73.9. Shin DH, Yu HS, Park JH, Shin JH, Kim SJ. Recently occurring adult tetanus in Korea: emphasis on immunization and awareness of tetanus. J Korean Med Sci. 2003. 18:11–16.10. Pickering LK, Baker CJ, Kimberlin DW, Long SS. Diphtheria: Red Book: 2009 report of the Committee on Infectious Diseases. 2009. 28th ed. Elk Grove Village: American Academy of Pediatrics;280–283.11. Kang JH, Lee SY, Kim HM, Park JS, Ma SH. The assessment of adult Td vaccine usefulness. KFDA. 2007. 32–33.12. Lee SY, Kwak GY, Nam CH, Kim JH, Hur JK, Lee KY, Park JS, Kim HM, Kang JH. Immunogenicity and safety of diphtheria-tetanus vaccine in pre-adolescent and adolescent South Koreans. Vaccine. 2009. 27:3209–3212.13. Choi JH, Choo EJ, Huh A, Choi SM, Eom JS, Lee JS, Park SH, Kang JH. Immunogenicity and safety of diphtheria-tetanus vaccine in adults. J Korean Med Sci. 2010. 25:1727–1732.14. Gil A, Dal-Ré R, González A, Lasheras L, Aguilar L, del Rey J. Immunogenicity and safety of a tetanus-diphtheria vaccine (adult type): clinical trial in adults. Med Clin (Barc). 1995. 104:126–129.15. Turnbull FM, Heath TC, Jalaludin BB, Burgess MA, Ramalho AC. A randomized trial of two acellular pertussis vaccines (dTpa and pa) and a licensed diphtheria-tetanus vaccine (Td) in adults. Vaccine. 2000. 19:628–636.16. Bartels I, Jüngert J, Lugauer S, Stehr K, Heininger U. Immunogenicity and reactogenicity of a single dose of a diphtheria-tetanus: acellular pertussis component vaccine (DTaP) compared to a diphtheria: tetanus toxoid (Td) and a diphtheria toxoid vaccine (d) in adults. Vaccine. 2001. 19:3137–3145.17. Mark A, Carlsson RM, Granström M. Subcutaneous versus intramuscular injection for booster DT vaccination of adolescents. Vaccine. 1999. 17:2067–2072.18. Blennow M, Granström M, Strandell A. Adverse reactions after diphtheria-tetanus booster in 10-year-old schoolchildren in relation to the type of vaccine given for the primary vaccination. Vaccine. 1994. 12:427–430.19. Southern J, Andrews N, Burrage M, Miller E. Immunogenicity and reactogenicity of combined acellular pertussis/tetanus/low dose diphtheria vaccines given as a booster to UK teenagers. Vaccine. 2005. 23:3829–3835.20. Relyveld EH, Bizzini B, Gupta RK. Rational approaches to reduce adverse reactions in man to vaccines containing tetanus and diphtheria toxoids. Vaccine. 1998. 16:1016–1023.21. Jackson LA, Yu O, Belongia EA, Hambidge SJ, Nelson J, Baxter R, Naleway A, Gay C, Nordin J, Baggs J, et al. Frequency of medically attended adverse events following tetanus and diphtheria toxoid vaccine in adolescents and young adults: a Vaccine Safety Datalink Study. BMC Infect Dis. 2009. 9:165.22. Lodolce AE. Shortened interval between tetanus vaccines. Ann Pharmacother. 2012. 46:884–888.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Phase III Study to Evaluate the Immunogenicity and Safety of GC1107 (Adult Tetanus Diphtheria Vaccine) in Healthy Adults
- Immunogenicity and Safety of a Newly Developed Tetanus-Diphtheria Toxoid (Td) in Healthy Korean Adolescents: a Multi-center, Randomized, Doubleblind, Active-Controlled Phase 3 Trial
- The Need of Td Vaccination According to the Changes of Tetanus and Diphtheria Immunity
- Immunogenicity and Safety of Diphtheria-tetanus Vaccine in Adults
- The immunogenicity and reactogenicity of Td booster vaccination in Korean preadolescents, aged with 11-12 years old