J Korean Med Sci.
2010 Dec;25(12):1727-1732. 10.3346/jkms.2010.25.12.1727.
Immunogenicity and Safety of Diphtheria-tetanus Vaccine in Adults
- Affiliations
-
- 1Department of Internal Medicine, The Catholic University of Korea, Seoul, Korea.
- 2Department of Internal Medicine, Soonchunhyang University College of Medicine, Bucheon, Korea.
- 3National Health Insurance Corporation Ilsan Hostpital, Goyang, Korea.
- 4Department of Internal Medicine, Hallym University Medical College, Chuncheon, Korea.
- 5Department of Pediatrics, The Catholic University of Korea, Seoul, Korea. kjhan@catholic.ac.kr
- KMID: 1792895
- DOI: http://doi.org/10.3346/jkms.2010.25.12.1727
Abstract
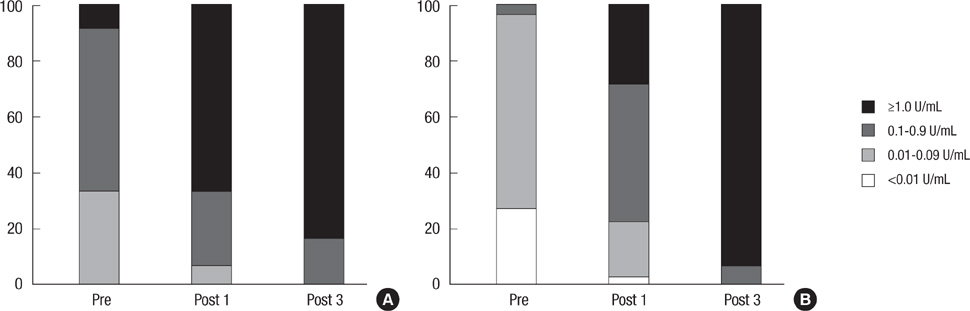
- This study was conducted to evaluate the immunogenicity and safety of diphtheria-tetanus (Td) vaccine in adults over 40 yr old who had never received a diphtheria-tetanus-pertussis (DTP) vaccination. A total of 242 subject completed three-doses of Td vaccination and subsequent assays for immunogenicity. Before vaccination, 33.9% and 96.7% participants showed antibody levels of diphtheria and tetanus, respectively, which were below protective level (<0.1 U/mL). After the first dose of Td vaccine, 92.6% and 77.6% of subjects gained protective antibody concentrations (> or =0.1 U/mL) for diphtheria and tetanus, with an increase to 99.6% and 100% after the third dose. Local and systemic adverse events occurred in 37.9% and 15.5% of the subjects. No serious adverse event requiring an unscheduled hospital visit occurred. In conclusion, three-doses of Td vaccination to unimmunized adults are safe and effective in inducing protective immunity against diphtheria and tetanus.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
A Phase III Study to Evaluate the Immunogenicity and Safety of GC1107 (Adult Tetanus Diphtheria Vaccine) in Healthy Adults
Jacob Lee, Jung-Hyun Choi, Seong-Heon Wie, Sun Hee Park, Su-Mi Choi, Mi Suk Lee, Tae Hyong Kim, Hyo-Jin Lee, Jin Han Kang
J Korean Med Sci. 2019;34(4):. doi: 10.3346/jkms.2019.34.e31.Vaccination of Hematopoietic Stem Cell Transplantation Recipients: Perspective in Korea
Dong-Gun Lee
Infect Chemother. 2013;45(3):272-282. doi: 10.3947/ic.2013.45.3.272.Vaccination necessary for Korean adults
Hee Jin Cheong
J Korean Med Assoc. 2011;54(12):1289-1296. doi: 10.5124/jkma.2011.54.12.1289.
Reference
-
1. Turner TB, Velasco-Joven EA, Prudovsky S. Studies on the prophylaxis and treatment of tetanus. II. Studies pertaining to treatment. Bull Johns Hopkins Hosp. 1958. 102:71–84.2. Cain HD, Falco FG. Recurrent tetanus. Calif Med. 1962. 97:31–33.3. Vitek CR, Wharton M. Plotkin SA, Orenstein WA, Offit PA, editors. Diphtheria toxoid. Vaccines. 2008. 5th ed. Philadelphia: WB Saunders Co;139–156.
Article4. Wassilak SG, Roper MH, Kretsinger K, Orenstein WA. Plotkin SA, Orenstein WA, Offit PA, editors. Tetanus toxoid. Vaccines. 2008. 5th ed. Philadelphia: WB Saunders Co;805–840.
Article5. Gergen PJ, McQuillan GM, Kiely M, Ezzati-Rice TM, Sutter RW, Virella G. A population-based serologic survey of immunity to tetanus in the United States. N Engl J Med. 1995. 332:761–766.
Article6. Broder KR, Cortese MM, Iskander JK, Kretsinger K, Slade BA, Brown KH, Mijalski CM, Tiwari T, Weston EJ, Cohn AC, Srivastava PU, Moran JS, Schwartz B, Murphy TV. Advisory Committee on Immunization Practices (ACIP). Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006. 55:RR-3. 1–34.
Article7. Kang JH. The need of Td vaccination according to the changes of tetanus and diphtheria Immunity. J Korean Med Assoc. 2008. 51:127–136.
Article8. Kang JH, Hur JK, Kim JH, Lee KI, Park SE, Ma SH, Lee MS, Baek SY, Hong SH, Min HK. Age related seroepidemiological study of diphtheria among Koreans. Korean J Infect Dis. 2000. 32:1–7.9. Kang JH, Hur JK, Kim JH, Lee KI, Park SE, Ma SH, Lee MS, Ban SJ, Hong SH, Cho DH, Lee SH. Age related serosurvey of immunity to tetanus in Korean populations. Korean J Infect Dis. 2001. 33:104–111.10. Galazka AM, Robertson SE, Oblapenko GP. Resurgence of diphtheria. Eur J Epidemiol. 1995. 11:95–105.
Article11. Hardy IR, Dittmann S, Sutter RW. Current situation and control strategies for resurgence of diphtheria in the newly independent states of former Soviet Union. Lancet. 1996. 347:1739–1744.12. MacGregor RR. Mandell GL, Bennett JE, Dolin P, editors. Corynebacterium diphtheriae. Principles and Practice of Infectious Diseases. 2009. 7th ed. Philadelphia: Elsevier;2687–2693.
Article13. Lee SY, Kwak GY, Mok HR, Kim JH, Hur JK, Lee KI, Park JS, Ma SH, Kim HM, Kang JH. The immunogenicity and reactogenicity of Td booster vaccination in Korean preadolescence, aged with 11-12 years old. Korean J Pediatr. 2008. 51:1185–1190.14. Lee SY, Kwak GY, Nam CH, Kim JH, Hur JK, Lee KY, Park JS, Kim HM, Kang JH. Immunogenicity and safety of diphtheria-tetanus vaccine in pre-adolescent and adolescent South Koreans. Vaccine. 2009. 27:3209–3212.
Article15. Shin DH, Yu HS, Park JH, Shin JH, Kim SJ. Recently occurring adult tetanus in Korea: emphasis on immunization and awareness of tetanus. J Korean Med Sci. 2003. 18:11–16.
Article16. Yun YH, Park HJ, Yu SW, Kwon SB, Minn YK, Cho SJ, Kwon KH. A case of polyneuropathy suggesting diphtheritic neuropathy. J Korean Neurol Assoc. 2005. 23:288–289.17. Brennan M, Vitek C, Strebel P, Wattigney W, Bisgard K, Brisgalov S, Bragina V, Pyanikh V, Wharton M. How many doses of diphtheria toxoid are required for protection in adults? Results of a case-control study among 40- to 49-year-old adults in the Russian Federation. J Infect Dis. 2000. 181:Suppl 1. S193–S196.
Article18. Sutter RW, Hardy IR, Kozlova IA, Tchoudnaia LM, Gluskevich TG, Marievsky V, Deforest A, Wharton M. Immunogenicity of tetanus-diphtheria (Td) among Ukrainian adults: Implications for diphtheria control in the newly independent states of the former Soviet Union. J Infect Dis. 2000. 181:Suppl 1. S197–S202.19. Khetsuriani N, Music S, Deforest A, Sutter RW. Evaluation of a single dose of diphtheria toxoid among adults in the Republic of Georgia, 1995: immunogenicity and adverse reactions. J Infect Dis. 2000. 181:Suppl 1. S208–S212.
Article20. Weckx LY, Divino-Goes K, Lihama DM, Carraro E, Bellei N, Granato CF, Moraes-Pinto MI. Effect of a single tetanus-diphtheria vaccine dose on the immunity of elderly people in S㯠Paulo, Brazil. Braz J Med Biol Res. 2006. 39:519–523.21. Vilella A, Dal-Re R, Simo D, García-Corbeira P, Diego P, Bayas JM. Reactogenicity profile of tetanus-diphtheria (adult-type) vaccine: results of a naturalistic study performed at an adult vaccination center. J Clin Pharmacol. 2000. 40:1267–1273.22. Ciofi degli Atti ML, Salmaso S, Cotter B, Gallo G, Alfarone G, Pinto A, Bella A, von Hunolstein C. Reactogenicity and immunogenicity of adult versus paediatric diphtheria and tetanus booster dose at 6 years of age. Vaccine. 2001. 20:74–79.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tetanus–diphtheria–acellular pertussis vaccination for adults: an update
- Antltoxln response to diphtheria and tetananus vaccine
- A Bordetella pertussis proteoliposome induces protection in mice without affecting the immunogenicity of diphtheria and tetanus toxoids in a trivalent formulation
- Rates of Adverse Reactions Associated with Modified DPT Vaccine in Korean Infants and Children
- The immunogenicity and safety of three-component DTaP vaccine in Korean infants