J Korean Med Sci.
2021 Dec;36(49):e313. 10.3346/jkms.2021.36.e313.
Immunogenicity and Safety of a Newly Developed Tetanus-Diphtheria Toxoid (Td) in Healthy Korean Adolescents: a Multi-center, Randomized, Doubleblind, Active-Controlled Phase 3 Trial
- Affiliations
-
- 1Department of Pediatrics, Eunpyeong St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Pediatrics, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Department of Pediatrics, Hanil General Hospital, Seoul, Korea
- 4Department of Pediatrics, Nowon Eulji University Hospital, Eulji University School of Medicine, Seoul, Korea
- 5Department of Pediatrics, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea
- 6Department of Pediatrics, Daejeon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 7Department of Pediatrics, Korea Cancer Center Hospital, Seoul, Korea
- 8Department of Pediatrics, Changwon Fatima Hospital, Changwon, Korea
- 9Department of Pediatrics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 10Department of Pediatrics, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2523483
- DOI: http://doi.org/10.3346/jkms.2021.36.e313
Abstract
- Background
Although the combination tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) is recommended at adolescence in developed countries, the tetanus and diphtheria toxoid vaccine (Td), which is less costly, is recommended instead in some parts of the world. A new Td, BR-TD-1001, was developed by a Korean manufacturer for distribution to endemic regions and for use in the initial step of novel Tdap development.
Methods
This phase 3, randomized, double-blind, multi-center trial, conducted in Korea, aimed to evaluate the immunogenicity and safety of BR-TD-1001. Healthy children aged 10 to 12 years were randomized 1:1 to receive either BR-TD-1001 or the control Td (Td-pur, GlaxoSmithKline). Antibodies were measured using enzyme-linked immunosorbent assay.
Results
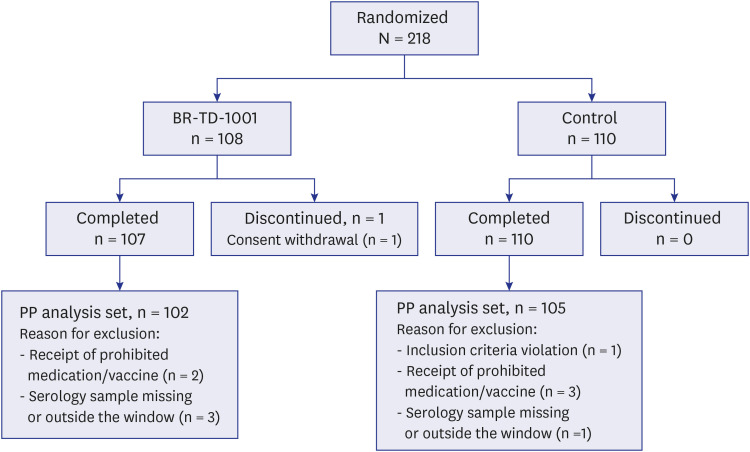
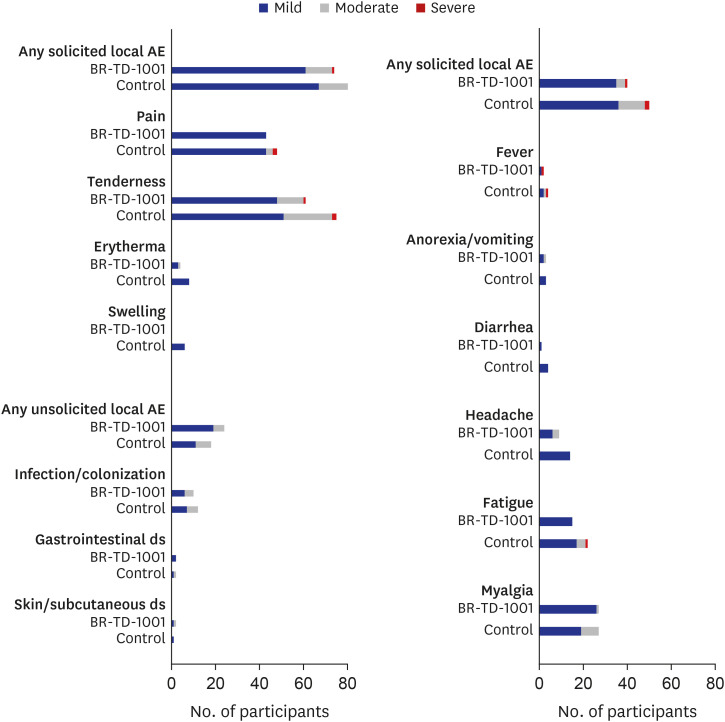
A total of 218 subjects (BR-TD-1001, n = 108; control, n = 110) were enrolled and included in the safety analysis. Vaccine-mediated antibody responses were similar in both groups. We confirmed the non-inferiority of BR-TD-1001 against the control, Td; 100% of both groups achieved seroprotection against diphtheria and tetanus. Furthermore, there was no significant difference between groups in the proportion of participants who demonstrated boost responses against diphtheria and tetanus toxoids. The incidence of solicited local and systemic adverse events (AEs), unsolicited AEs, and serious AEs did not differ significantly between groups.
Conclusion
The BR-TD-1001 satisfied the immunological non-inferiority criterion against diphtheria and tetanus, with a clinically acceptable safety profile.
Keyword
Figure
Reference
-
1. Kim JH, Bae W, Kim J, Hwang ES. An urgent need for global preparedness against the reemergence of “forgotten” infectious disease in Korea. J Korean Med Sci. 2018; 33(17):e125. PMID: 29686596.
Article2. Mungall BA, Kim H, Oh KB. A systematic review of the burden of pertussis in South Korea. Hum Vaccin Immunother. 2021; 17(6):1747–1756. PMID: 33412085.
Article3. Clarke KE, MacNeil A, Hadler S, Scott C, Tiwari TS, Cherian T. Global epidemiology of diphtheria, 2000–2017. Emerg Infect Dis. 2019; 25(10):1834–1842. PMID: 31538559.4. Our World Data. Tetanus. Updated 2021. Accessed July 26, 2021. https://ourworldindata.org/tetanus .5. Ryu S, Kim JJ, Chen MY, Jin H, Lee HK, Chun BC. Outbreak investigation of pertussis in an elementary school: a case-control study among vaccinated students. Clin Exp Vaccine Res. 2018; 7(1):70–75. PMID: 29399582.
Article6. Alimohamadi Y, Zahraei SM, Karami M, Yaseri M, Lotfizad M, Holakouie-Naieni K. Alarrm thresholds for pertussis outbreaks in Iran: national data analysis. Osong Public Health Res Perspect. 2020; 11(5):309–318. PMID: 33117636.7. Desai S, Scobie HM, Cherian T, Goodman T. Expert Group on the Use of Td vaccine in Childhood. Use of tetanus-diphtheria (Td) vaccine in children 4–7 years of age: World Health Organization consultation of experts. Vaccine. 2020; 38(21):3800–3807. PMID: 31983584.8. WHO. Pertussis vaccines: WHO position paper, August 2015--recommendations. Vaccine. 2016; 34(12):1423–1425. PMID: 26562318.9. Havers FP, Moro PL, Hunter P, Hariri S, Bernstein H. Use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines: Updated recommendations of the advisory committee on immunization practices – United States, 2019. MMWR Morb Mortal Wkly Rep. 2020; 69(3):77–83. PMID: 31971933.
Article10. World Health Organization. Global market study. Diphtheria and tetanus containing vaccines. Updated 2021. Accessed July 26, 2021. https://www.who.int/immunization/programmes_systems/procurement/mi4a/platform/WHO_DT_global_market_study.pdf .11. Centers for Disease Control and Prevention. CDC vaccine price list. Updated 2021. Accessed July 26, 2021. https://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price- list/index.html .12. Park HJ, Kim SJ, Song R, Chen J, Kim JH, Devadiga R, et al. A 6-year prospective, observational, multi-center post-marketing surveillance of the safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in Korea. J Korean Med Sci. 2019; 34(12):e105. PMID: 30940999.
Article13. Lee J, Choi JH, Wie SH, Park SH, Choi SM, Lee MS, et al. A phase III study to evaluate the immunogenicity and safety of GC1107 (adult tetanus diphtheria vaccine) in healthy adults. J Korean Med Sci. 2019; 34(4):e31. PMID: 30686952.
Article15. Tiwari TS, Wharton M. Diphtheria toxoid. Plotkin SA, Orentein WA, Offit PA, Edwards KM, editors. Plotkin's Vaccines. 7th ed. Philadelphia, PA: Elsevier;2018. p. 261–275.16. Roper MH, Wassilak SG, Scobie HM, Ridpath AD, Orenstein WA. Tetanus toxoid. Plotkin SA, Orsentein WA, Offit PA, Edwards KM, editors. Plotkin's Vaccines. 7th ed. Philadelphia, PA: Elsevier;2018. p. 1052–1080.17. Sung H, Jang MJ, Bae EY, Han SB, Kim JH, Kang JH, et al. Seroepidemiology of tetanus in Korean adults and adolescents in 2012. J Infect Chemother. 2014; 20(7):397–400. PMID: 24802766.
Article18. World Health Organization. Immunological basis for immunization: module 2: diphtheria-update 2009, update 2009. Updated 2009. Accessed July 26, 2021. https://apps.who.int/iris/handle/10665/44094 .19. World Health Organization. The immunological basis for immunization series: module 3: tetanus, update 2018. Updated 2018. Accessed July 26, 2021. https://apps.who.int/iris/handle/10665/275340 .20. Medical dictionary for regulatory activities. Updated 2018. Accessed July 26, 2021. http://meddra.org .21. Ministry of Korean Food and Drug Safety. Multi-national Vaccine Clinical Trial Protocol. 1st ed. Cheongju, Korea: Ministry of Korean Food and Drug Safety;2016. p. 377–445.22. Lloyd JC, Haber P, Mootrey GT, Braun MM, Rhodes PH, Chen RT, et al. Adverse event reporting rates following tetanus-diphtheria and tetanus toxoid vaccinations: data from the Vaccine Adverse Event Reporting System (VAERS), 1991–1997. Vaccine. 2003; 21(25-26):3746–3750. PMID: 12922107.
Article23. Baraff LJ, Manclark CR, Cherry JD, Christenson P, Marcy SM. Analyses of adverse reactions to diphtheria and tetanus toxoids and pertussis vaccine by vaccine lot, endotoxin content, pertussis vaccine potency and percentage of mouse weight gain. Pediatr Infect Dis J. 1989; 8(8):502–507. PMID: 2771530.24. Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007; 357(19):1903–1915. PMID: 17989383.
Article25. World Health Organization. Global Immunization 1980–2019. Global Coverage from 3 doses of DTP containing vaccines at 85% in 2019. Updated 2020. Accessed July 27, 2021. https://www.who.int/data/gho/data/themes/immunization .26. Rahman MR, Islam K. Massive diphtheria outbreak among Rohingya refugees: lessons learnt. J Travel Med. 2019; 26(1):tay122.
Article27. Lodeiro-Colatosti A, Reischl U, Holzmann T, Hernández-Pereira CE, Rísquez A, Paniz-Mondolfi AE. Diphtheria outbreak in Amerindian communities, Wonken, Venezuela, 2016–2017. Emerg Infect Dis. 2018; 24(7):1340–1344. PMID: 29912686.
Article28. Korea Disease Control and Prevention Agency. Infections disease portal [In Korean]. Updated 2021. Accessed July 27, 2021. http://www.kdca.go.kr/npt/biz/npp/ist/simple/simplePdStatsMain.do .29. Cherry JD. The 112-year odyssey of pertussis and pertussis vaccines-mistakes made and implication for the future. J Pediatric Infect Dis Soc. 2019; 8(4):334–341. PMID: 30793754.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Immunogenicity and Safety of the New Tetanus-Reduced Diphtheria (Td) Vaccines (GC1107) in Healthy Korean Adolescents: A Phase II, Double-Blind, Randomized, Multicenter Clinical Trial
- Immunogenicity and Safety of Diphtheria-tetanus Vaccine in Adults
- The immunogenicity and reactogenicity of Td booster vaccination in Korean preadolescents, aged with 11-12 years old
- The Need of Td Vaccination According to the Changes of Tetanus and Diphtheria Immunity
- Antltoxln response to diphtheria and tetananus vaccine