J Korean Med Sci.
2011 Jun;26(6):747-752. 10.3346/jkms.2011.26.6.747.
Morphine Attenuates Endothelial Cell Adhesion Molecules Induced by the Supernatant of LPS-Stimulated Colon Cancer Cells
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Ansan, Korea. minware2@lycos.co.kr
- 2Department of Biomedical Sciences, Korea University College of Medicine, Seoul, Korea.
- KMID: 1785959
- DOI: http://doi.org/10.3346/jkms.2011.26.6.747
Abstract
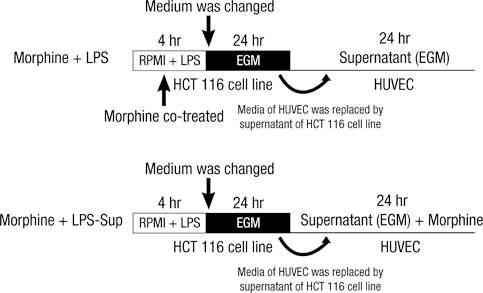
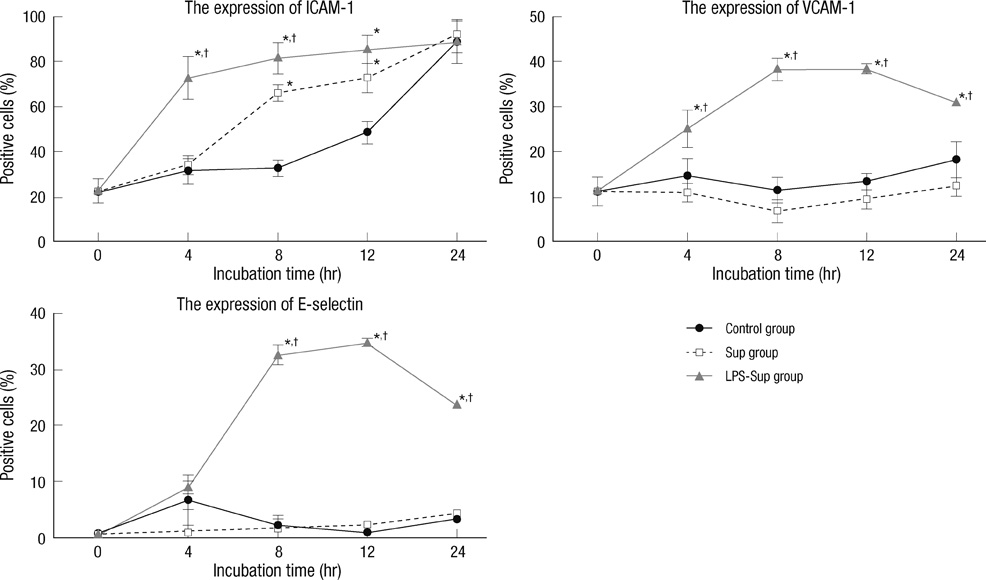
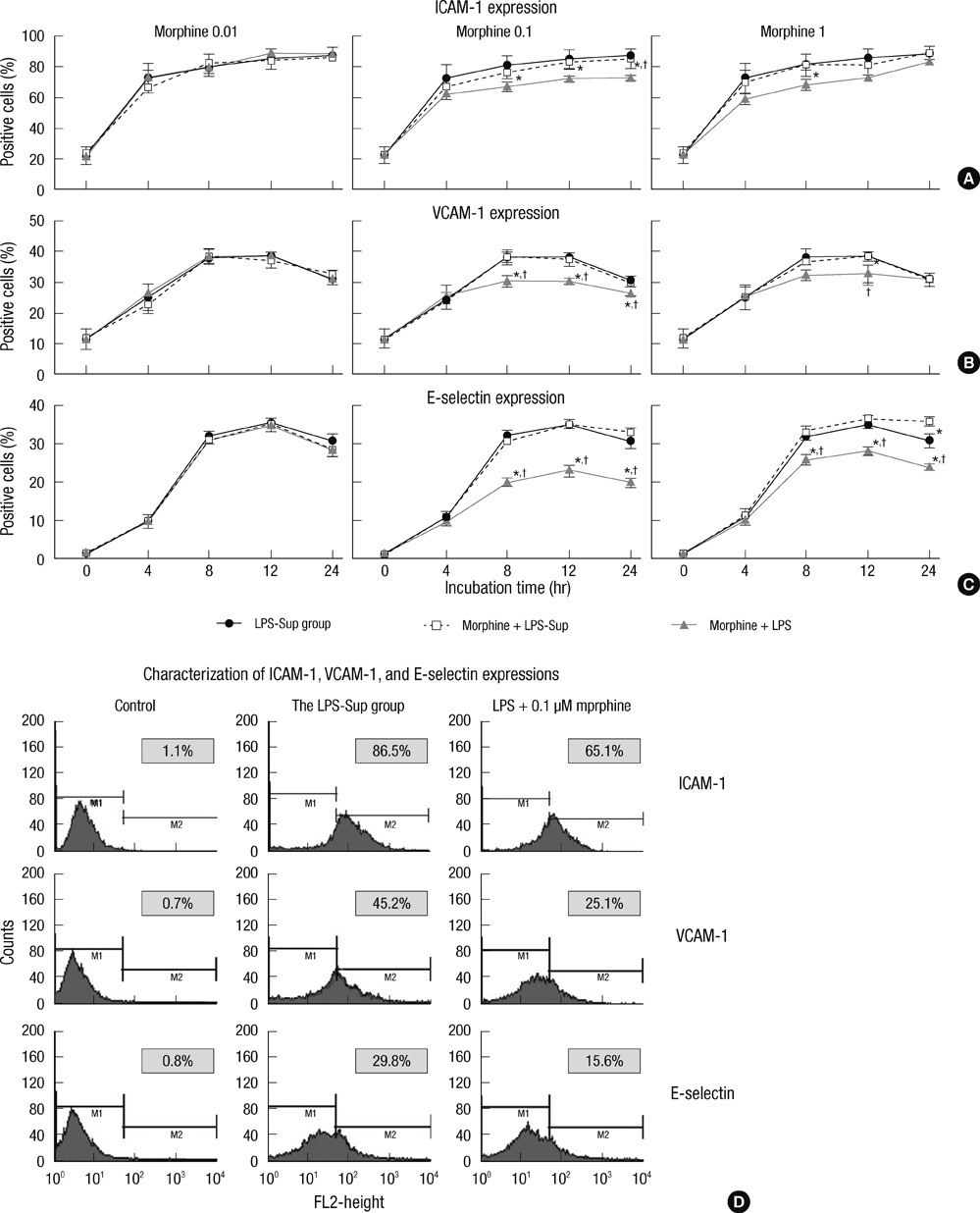
- A large reservoir of bacterial lipopolysaccharide (LPS) is available in the colon and this could promote colon cancer metastasis by enhancing tumor cell adhesion, intravasation, and extravasation. Furthermore, adhesion molecules like ICAM-1, VCAM-1, and E-selectin play important roles in the adhesion of tumor cells to endothelium. This study was designed to determine whether morphine can attenuate the expressions of adhesion molecules up-regulated by the supernatant of LPS-stimulated HCT 116 colon cancer cells (LPS-Sup). In this study, we divided to three groups by cell-growth medium of human umbilical vascular endothelial cells (HUVECs): the control group was incubated in growth factor-free endothelial medium, the Sup group was incubated in the supernatant of HCT 116 cells (Sup), and the LPS-Sup group was incubated in LPS-Sup. To observe effect of morphine to the adhesion molecules expressions in the LPS-Sup group, we co-treated morphine with LPS or added it to LPS-Sup. Adhesion molecule expressions on HUVECs in all three groups were measured during incubation period. Consquentially, ICAM-1, VCAM-1, and E-selectin expressions on HUVECs were significantly lower when morphine was co-treated with LPS than not co-treated. Thus, we suggest that morphine affects the expressions of adhesion molecules primarily by attenuating LPS stimuli on tumor cells.
Keyword
MeSH Terms
-
Cell Adhesion Molecules/*metabolism
Cell Line, Tumor
Cell Survival/drug effects
Colonic Neoplasms/*metabolism
E-Selectin/metabolism
Endothelial Cells/drug effects/metabolism
Endothelium, Vascular/cytology
Humans
Intercellular Adhesion Molecule-1/metabolism
Lipopolysaccharides/toxicity
Morphine/*pharmacology
Vascular Cell Adhesion Molecule-1/metabolism
Figure
Cited by 1 articles
-
Hydromorphone attenuates intercellular adhesion molecule-1 expressions induced by lipopolysaccharide on HCT-116 human colon cancer cells
Jae Jin Lee, Woon Young Kim, Ji Hye Um, Too Jae Min
Korean J Anesthesiol. 2014;67(Suppl):S124-S126. doi: 10.4097/kjae.2014.67.S.S124.
Reference
-
1. Lafrenie RM, Buchanan MR, Orr FW. Adhesion molecules and their role in cancer metastasis. Cell Biophys. 1993. 23:3–89.2. Kobayashi H, Boelte KC, Lin PC. Endothelial cell adhesion molecules and cancer progression. Curr Med Chem. 2007. 14:377–386.3. Szajnik M, Szczepanski MJ, Czystowska M, Elishaev E, Mandapathil M, Nowak-Markwitz E, Spaczynski M, Whiteside TL. TLR4 signaling induced by lipopolysaccharide or paclitaxel regulates tumor survival and chemoresistance in ovarian cancer. Oncogene. 2009. 28:4353–4363.4. Grandel U, Heygster D, Sibelius U, Fink L, Sigel S, Seeger W, Grimminger F, Hattar K. Amplification of lipopolysaccharide-induced cytokine synthesis in non-small cell lung cancer/neutrophil cocultures. Mol Cancer Res. 2009. 7:1729–1735.5. Sasamura T, Nakamura S, Iida Y, Fujii H, Murata J, Saiki I, Nojima H, Kuraishi Y. Morphine analgesia suppresses tumor growth and metastasis in a mouse model of cancer pain produced by orthotopic tumor inoculation. Eur J Pharmacol. 2002. 441:185–191.6. Harimaya Y, Koizumi K, Andoh T, Nojima H, Kuraishi Y, Saiki I. Potential ability of morphine to inhibit the adhesion, invasion and metastasis of metastatic colon 26-L5 carcinoma cells. Cancer Lett. 2002. 187:121–127.7. Wang TL, Chang H, Hung CR, Tseng YZ. Morphine preconditioning attenuates neutrophil activation in rat models of myocardial infarction. Cardiovasc Res. 1998. 40:557–563.8. Wang TL, Chang H, Hung CR, Tseng YZ. Attenuation of neutrophil and endothelial activation by intravenous morphine in patients with acute myocardial infarction. Am J Cardiol. 1997. 80:1532–1535.9. Weber NC, Kandler J, Schlack W, Grueber Y, Fradorf J, Preckel B. Intermitted pharmacologic pretreatment by xenon, isoflurane, nitrous oxide, and the opioid morphine prevents tumor necrosis factor alpha-induced adhesion molecule expression in human umbilical vein endothelial cells. Anesthesiology. 2008. 108:199–207.10. Penson RT, Joel SP, Gloyne A, Clark S, Slevin ML. Morphine analgesia in cancer pain: role of the glucuronides. J Opioid Manag. 2005. 1:83–90.11. Dershwitz M, Walsh JL, Morishige RJ, Connors PM, Rubsamen RM, Shafer SL, Rosow CE. Pharmacokinetics and pharmacodynamics of inhaled versus intravenous morphine in healthy volunteers. Anesthesiology. 2000. 93:619–628.12. Simiantonaki N, Jayasinghe C, Kirkpatrick CJ. Effect of pro-inflammatory stimuli on tumor cell-mediated induction of endothelial cell adhesion molecules in vitro. Exp Mol Pathol. 2002. 73:46–53.13. Vidal-Vanaclocha F, Alvarez A, Asumendi A, Urcelay B, Tonino P, Dinarello CA. Interleukin 1 (IL-1)-dependent melanoma hepatic metastasis in vivo; increased endothelial adherence by IL-1-induced mannose receptors and growth factor production in vitro. J Natl Cancer Inst. 1996. 88:198–205.14. Andrews EJ, Wang JH, Winter DC, Laug WE, Redmond HP. Tumor cell adhesion to endothelial cells is increased by endotoxin via an upregulation of beta-1 integrin expression. J Surg Res. 2001. 97:14–19.15. Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995. 9:899–909.16. Smirnova MG, Guo L, Birchall JP, Pearson JP. LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell Immunol. 2003. 221:42–49.17. Wang L, Liu Q, Sun Q, Zhang C, Chen T, Cao X. TLR4 signaling in cancer cells promotes chemoattraction of immature dendritic cells via autocrine CCL20. Biochem Biophys Res Commun. 2008. 366:852–856.18. Takeda K, Fujii N, Nitta Y, Sakihara H, Nakayama K, Rikiishi H, Kumagai K. Murine tumor cells metastasizing selectively in the liver: ability to produce hepatocyte-activating cytokines interleukin-1 and/or -6. Jpn J Cancer Res. 1991. 82:1299–1308.19. Thornton P, McColl BW, Cooper L, Rothwell NJ, Allan SM. Interleukin-1 drives cerebrovascular inflammation via MAP kinase-independent pathways. Curr Neurovasc Res. 2010. 7:330–340.20. Page GG, Ben-Eliyahu S, Yirmiya R, Liebeskind JC. Morphine attenuates surgery-induced enhancement of metastatic colonization in rats. Pain. 1993. 54:21–28.21. Kanno J, Wakikawa A, Utsuyama M, Hirokawa K. Effect of restraint stress on immune system and experimental B16 melanoma metastasis in aged mice. Mech Ageing Dev. 1997. 93:107–117.22. Min TJ, Kim JI, Kim JH, Noh KH, Kim TW, Kim WY, Lee YS, Park YC. Morphine postconditioning attenuates ICAM-1 expression on endothelial cells. J Korean Med Sci. 2011. 26:290–296.23. Ohno O, Shima Y, Ikeda Y, Sakurai K, Watanabe K, Kawai Y, Umezawa K. Inhibition of cellular adhesion in human umbilical vein endothelial cells by NF-kappaB inhibitor DHMEQ under flow. Oncol Res. 2005. 15:189–197.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Allicin Reduces Adhesion Molecules and NO Production Induced by gamma irradiation in Human Endothelial Cells
- Heparin Attenuates the Expression of TNF alpha-induced Cerebral Endothelial Cell Adhesion Molecule
- A New Endothelial Molecule Involved in Melanoma Cell Binding to Human Dermal Microvascular Endothelial Cells
- GPR40 Agonism Modulates Inflammatory Reactions in Vascular Endothelial Cells
- Effects of mixed leukocyte reaction, hydrocortisone and cyclosporine on expression of leukocyte adhesion molecules by endothelial and mesangial cells