Diabetes Metab J.
2022 May;46(3):506-511. 10.4093/dmj.2021.0092.
GPR40 Agonism Modulates Inflammatory Reactions in Vascular Endothelial Cells
- Affiliations
-
- 1BK21 Graduate Program, Department of Biomedical Sciences, Korea University College of Medicine, Seoul, Korea
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea
- KMID: 2530189
- DOI: http://doi.org/10.4093/dmj.2021.0092
Abstract
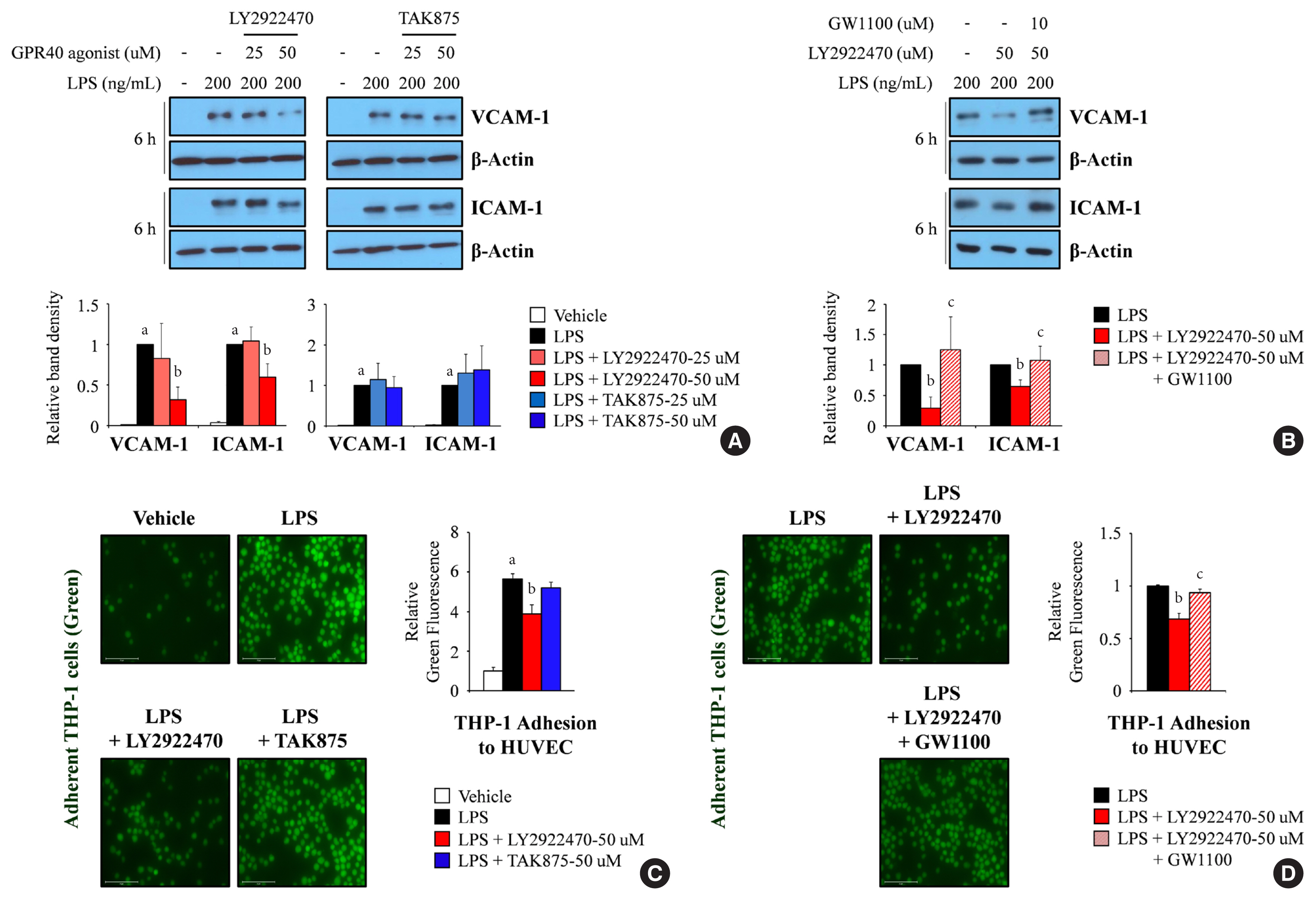
- Endothelial dysfunction is strongly linked with inflammatory responses, which can impact cardiovascular disease. Recently, G protein-coupled receptor 40 (GPR40) has been investigated as a modulator of metabolic stress; however, the function of GPR40 in vascular endothelial cells has not been reported. We analyzed whether treatment of GPR40-specific agonists modulated the inflammatory responses in human umbilical vein endothelial cells (HUVECs). Treatment with LY2922470, a GPR40 agonist, significantly reduced lipopolysaccharide (LPS)-mediated nuclear factor-kappa B (NF-κB) phosphorylation and movement into the nucleus from the cytosol. However, treatment with another GPR40 agonist, TAK875, did not inhibit LPS-induced NF-κB activation. LPS treatment induced expression of adhesion molecules vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) and attachment of THP-1 cells to HUVECs, which were all decreased by LY2922470 but not TAK875. Our results showed that ligand-dependent agonism of GPR40 is a promising therapeutic target for overcoming inflammatory reactions in the endothelium.
Keyword
Figure
Cited by 1 articles
-
AM1638, a GPR40-Full Agonist, Inhibited Palmitate-Induced ROS Production and Endoplasmic Reticulum Stress, Enhancing HUVEC Viability in an NRF2-Dependent Manner
Hwan-Jin Hwang, Joo Won Kim, SukHwan Yun, Min Jeong Park, Eyun Song, Sooyeon Jang, Ahreum Jang, Kyung Mook Choi, Sei Hyun Baik, Hye Jin Yoo
Endocrinol Metab. 2023;38(6):760-769. doi: 10.3803/EnM.2023.1774.
Reference
-
1. Rodrigues SF, Granger DN. Blood cells and endothelial barrier function. Tissue Barriers. 2015; 3:e978720.
Article2. Libby P. Inflammation in atherosclerosis. Nature. 2002; 420:868–74.
Article3. Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J Biol Chem. 2003; 278:11303–11.
Article4. Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003; 422:173–6.
Article5. Negoro N, Sasaki S, Mikami S, Ito M, Suzuki M, Tsujihata Y, et al. Discovery of TAK-875: a potent, selective, and orally bioavailable GPR40 agonist. ACS Med Chem Lett. 2010; 1:290–4.
Article6. Nagasumi K, Esaki R, Iwachidow K, Yasuhara Y, Ogi K, Tanaka H, et al. Overexpression of GPR40 in pancreatic beta-cells augments glucose-stimulated insulin secretion and improves glucose tolerance in normal and diabetic mice. Diabetes. 2009; 58:1067–76.7. Li M, Meng X, Xu J, Huang X, Li H, Li G, et al. GPR40 agonist ameliorates liver X receptor-induced lipid accumulation in liver by activating AMPK pathway. Sci Rep. 2016; 6:25237.
Article8. Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013; 38:1154–63.
Article9. Sun C, Li Y, Li X, Sun J. Agonism of Gpr40 protects the capacities of epidermal stem cells (ESCs) against ultraviolet-B (UV-B). Drug Des Devel Ther. 2020; 14:5143–53.10. Hwang HJ, Jung TW, Hong HC, Choi HY, Seo JA, Kim SG, et al. Progranulin protects vascular endothelium against atherosclerotic inflammatory reaction via Akt/eNOS and nuclear factor-κB pathways. PLoS One. 2013; 8:e76679.
Article11. Ho JD, Chau B, Rodgers L, Lu F, Wilbur KL, Otto KA, et al. Structural basis for GPR40 allosteric agonism and incretin stimulation. Nat Commun. 2018; 9:1645.
Article12. Mancini AD, Bertrand G, Vivot K, Carpentier E, Tremblay C, Ghislain J, et al. β-Arrestin recruitment and biased agonism at free fatty acid receptor 1. J Biol Chem. 2015; 290:21131–40.
Article13. Hauge M, Vestmar MA, Husted AS, Ekberg JP, Wright MJ, Di Salvo J, et al. GPR40 (FFAR1): combined Gs and Gq signaling in vitro is associated with robust incretin secretagogue action ex vivo and in vivo. Mol Metab. 2014; 4:3–14.
Article14. Lu Z, Li Y, Jin J, Zhang X, Hannun YA, Huang Y. GPR40/FFA1 and neutral sphingomyelinase are involved in palmitate-boosted inflammatory response of microvascular endothelial cells to LPS. Atherosclerosis. 2015; 240:163–73.
Article15. Wu J, Sun P, Zhang X, Liu H, Jiang H, Zhu W, et al. Inhibition of GPR40 protects MIN6 β cells from palmitate-induced ER stress and apoptosis. J Cell Biochem. 2012; 113:1152–8.
Article16. Lockyer JM, Colladay JS, Alperin-Lea WL, Hammond T, Buda AJ. Inhibition of nuclear factor-kappaB-mediated adhesion molecule expression in human endothelial cells. Circ Res. 1998; 82:314–20.17. Lopez-Franco O, Hernandez-Vargas P, Ortiz-Munoz G, Sanjuan G, Suzuki Y, Ortega L, et al. Parthenolide modulates the NF-kappaB-mediated inflammatory responses in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2006; 26:1864–70.18. Kaku K, Enya K, Nakaya R, Ohira T, Matsuno R. Efficacy and safety of fasiglifam (TAK-875), a G protein-coupled receptor 40 agonist, in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise: a randomized, double-blind, placebo-controlled, phase III trial. Diabetes Obes Metab. 2015; 17:675–81.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- AM1638, a GPR40-Full Agonist, Inhibited Palmitate- Induced ROS Production and Endoplasmic Reticulum Stress, Enhancing HUVEC Viability in an NRF2-Dependent Manner
- Inhibitory Effects of CD99-derived Peptide CD99CRIII3 on the Extravasation of Monocytes and Inflammatory Reactions in Contact Dermatitis Mouse Model
- Thrombospondin-1 and Inhibition of Tumor Growth
- In Vitro Culture of Endothelial Cell and Smooth Muscle Cell for Studying Vascular Diseases
- Up-regulation of Interleukin-8 by Vascular Endothelial Growth Factor in Vasculatures in vivo