J Korean Med Sci.
2010 Sep;25(9):1277-1283. 10.3346/jkms.2010.25.9.1277.
Acute Effects of Intravenous Administration of Pamidronate in Patients with Osteoporosis
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Inha University Hospital, Incheon, Korea. parkwon@inha.ac.kr
- 2Department of Occupational & Environmental Medicine, Inha University Hospital, Incheon, Korea.
- KMID: 1785905
- DOI: http://doi.org/10.3346/jkms.2010.25.9.1277
Abstract
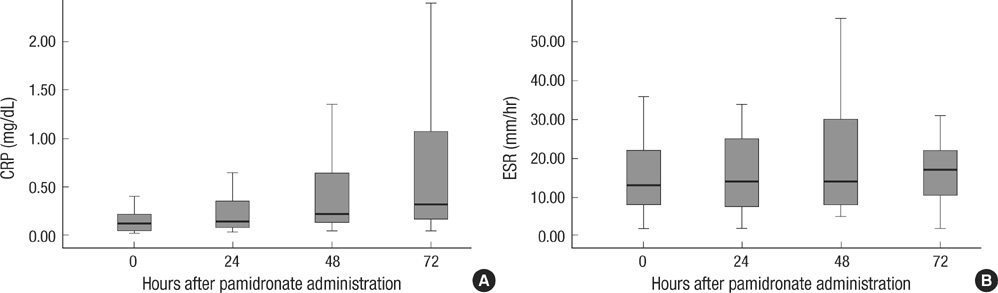
- We investigated acute effects of intermittent large dose bisphophonate therapy in osteoporotic patients. Peripheral blood mononuclear cells were incubated with alendronate (100 micrometer) for 18 hr, in vitro and cytokine expressions were measured by real-time RT-PCR. Pamidronate 30 mg was administered on 26 osteoporotic patients; and acute phase reactants, inflammatory cytokines and bone biomarkers were measured. The in vitro study showed significant increase in mRNA expression of IL-6, TNF-alpha and IFN-gamma. A notable rise in serum C-reactive protein (CRP) was observed over 3 days after pamidronate infusion (P=0.026). Serum levels of TNF-alpha, IL-6 and IFN-gamma were also significantly increased (P=0.009, 0.014, 0.035, respectively) and the increase in IL-6 levels were strongly correlated with CRP levels (P=0.04). Serum calcium and c-telopeptide levels rapidly decreased after the treatment (P=0.02, <0.001, respectively). This study showed that mRNA expression of inflammatory cytokines at peripheral blood mononuclear cells (PBMC) level were observed within 18 hr and marked elevation of inflammatory cytokines and acute phase reactants were demonstrated after pamidronate infusion at the dose for osteoporosis. Our studies confirmed that intermittent large dose aminobisphosphonate causes acute inflammation.
MeSH Terms
-
Acute-Phase Proteins/biosynthesis/genetics
Adult
Aged
Aged, 80 and over
Alendronate/pharmacology
Biological Markers/blood
Blood Cells/drug effects
Bone Density Conservation Agents/*administration & dosage
C-Reactive Protein/genetics/metabolism
Calcium/blood
Collagen Type I/blood
Diphosphonates/*administration & dosage
Female
Humans
Injections, Intravenous
Interferon-gamma/blood/genetics
Interleukin-6/blood/genetics
Male
Middle Aged
Osteoporosis/*drug therapy
Peptides/blood
RNA, Messenger/metabolism
Tumor Necrosis Factor-alpha/genetics/metabolism
Figure
Reference
-
1. Olson K, van Poznak C. Significance and impact of bisphosphate-induced acute phase responses. J Oncol Pharm Pract. 2007. 13:223–229.2. Rogers MJ, Gordon S, Benford HL, Coxon FP, Luckman SP, Monkkonen J, Frith JC. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000. 88:12 Suppl. 2961–2978.
Article3. Roelofs AJ, Thompson K, Gordon S, Rogers MJ. Molecular mechanisms of action of bisphosphonates: current status. Clin Cancer Res. 2006. 12:6222s–6230s.
Article4. Adami A, Bhalla AK, Dorizzi R, Montesanti F, Rosini S, Salvagno G, Lo Cascio V. The acute-phase response after bisphosphonate administration. Calcif Tissue Int. 1987. 41:326–331.
Article5. Bijvoet OL, Frijlink WB, Jie K, van der Linden H, Meijer CJ, Meijer H, Mulder H, van Paassen HC, Reitsma PH, te Velde J, de Vries E, van der Wey JP. APD in Paget's disease of bone. Role of the mononuclear phagocyte system? Arthritis Rheum. 1980. 23:1193–1204.
Article6. Schweitzer DH, Oostendorp-Van de Ruit M, Van der Pluijm G, Löwik CW, Papapoulos SE. Interleukin-6 and the acute phase response during treatment of patients with Paget's disease with the nitrogen-containing bisphosphonate dimethylaminohydroxypropylidene bisphosphonate. J Bone Miner Res. 1995. 10:956–962.
Article7. Sauty A, Pecherstorfer M, Zimmer-Roth I, Fioroni P, Juillerat L, Markert M, Ludwig H, Leuenberger P, Burckhardt P, Thiebaud D. Interleukin-6 and tumor necrosis factor alpha levels after bisphosphonates treatment in vitro and in patients with malignancy. Bone. 1996. 18:133–139.8. Thiebaud D, Sauty A, Burckhardt P, Leuenberger P, Sitzler L, Green JR, Kandra A, Zieschang J, Ibarra de Palacios P. An in vitro and in vivo study of cytokines in the acute-phase response associated with bisphosphonates. Calcif Tissue Int. 1997. 61:386–392.9. Santini D, Vincenzi B, Avvisati G, Dicuonzo G, Battistoni F, Gavasci M, Salerno A, Denaro V, Tonini G. Pamidronate induces modifications of circulating angiogenetic factors in cancer patients. Clin Cancer Res. 2002. 8:1080–1084.10. Leib ES, Lewiecki EM, Binkley N, Hamdy RC. International Society for Clinical Densitometry. Official positions of the International Society for Clinical Densitometry. J Clin Densitom. 2004. 7:1–6.
Article11. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001. 25:402–408.12. Moshage HJ, Roelofs HM, van Pelt JF, Hazenberg BP, van Leeuwen MA, Limburg PC, Aarden LA, Yap SH. The effect of interleukin-1, interleukin-6 and its interrelationship on the synthesis of serum amyloid A and C-reactive protein in primary cultures of adult human hepatocytes. Biochem Biophys Res Commun. 1988. 155:112–117.
Article13. Hewitt RE, Lissina A, Green AE, Slay ES, Price DA, Sewell AK. The bisphosphonate acute phase response: rapid and copious production of proinflammatory cytokines by peripheral blood gd T cells in response to aminobisphosphonates is inhibited by statins. Clin Exp Immunol. 2005. 139:101–111.
Article14. Green AE, Lissina A, Hutchinson SL, Hewitt RE, Temple B, James D, Boulter JM, Price DA, Sewell AK. Recognition of nonpeptide antigens by human V gamma 9V delta 2 T cells requires contact with cells of human origin. Clin Exp Immunol. 2004. 136:472–482.15. Miyagawa F, Tanaka Y, Yamashita S, Minato N. Essential requirement of antigen presentation by monocyte lineage cells for the activation of primary human gamma delta T cells by aminobisphosphonate antigen. J Immunol. 2001. 166:5508–5514.16. Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, Ebetino FH, Rogers MJ. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001. 296:235–242.17. Santini D, Vincenzi B, Caraglia M, Tonini G. A hitherto unreported high incidence of zoledronic acid-induced acute phase reaction in patients with cancer treatment-induced bone loss. Ann Oncol. 2007. 18:201–202.
Article18. Dicuonzo G, Vincenzi B, Santini D, Avvisati G, Rocci L, Battistoni F, Gavasci M, Borzomati D, Coppola R, Tonini G. Fever after zoledronic acid administration is due to increase in TNF-alpha and IL-6. J Interferon Cytokine Res. 2003. 23:649–654.19. Ferretti G, Fabi A, Carlini P, Papaldo P, Cordiali Fei P, Di Cosimo S, Salesi N, Giannarelli D, Alimonti A, Di Cocco B, D'Agosto G, Bordignon V, Trento E, Cognetti F. Zoledronic-acid-induced circulating level modifications of angiogenic factors, metalloproteinases and proinflammatory cytokines in metastatic breast cancer patients. Oncology. 2005. 69:35–43.
Article20. Santini D, Vincenzi B, Dicuonzo G, Avvisati G, Massacesi C, Battistoni F, Gavasci M, Rocci L, Tirindelli MC, Altomare V, Tocchini M, Bonsignori M, Tonini G. Zoledronic acid induces significant and long-lasting modifications of circulating angiogenic factors in cancer patients. Clin Cancer Res. 2003. 9:2893–2897.21. Chae BN, Hong EG, Lee SK, Chung YS, Lee KW, Kim HM. Cyclic pamidronate infusion in primary osteoporotic women. J Korean Soc Endocrinol. 2001. 16:221–230.22. Carbone LD, Warrington KJ, Barrow KD, Pugazhenthi M, Watsky MA, Somes G, Ingels J, Postlethwaite AE. Pamidronate infusion in patients with systemic sclerosis results in changes in blood mononuclear cell cytokine profiles. Clin Exp Immunol. 2006. 146:371–380.
Article23. Beatty G, Paterson Y. IFN-gamma-dependent inhibition of tumor angiogenesis by tumor-infiltrating CD4+ T cells requires tumor responsiveness to IFN-gamma. J Immunol. 2001. 166:2276–2282.24. Alonci A, Allegra A, Bellomo G, Quartarone E, Oteri G, Nastro E, Cicciù D, De Ponte FS, Musolino C. Patients with bisphosphonate-associated osteonecrosis of the jaw have unmodified levels of soluble vascular endothelial growth factor receptor 1. Leuk Lymphoma. 2007. 48:1852–1854.
Article25. Shenker NG, Jawad AS. Bisphosphonates and osteonecrosis of the jaw. Rheumatology (Oxford). 2007. 46:1049–1051.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Author Response: Acute Effects of Intravenous Administration of Pamidronate in Patients with Osteoporosis
- Letter to the Editor: Acute Effects of Intravenous Administration of Pamidronate in Patients with Osteoporosis
- Effect of cyclic pamidronate administration on osteoporosis in children with β-thalassemia major: a single-center study
- Efficacy of pamidronate in pediatric osteosarcoma patients with low bone mineral density
- Biomechanical Analysis of the Effect of Pamidronate on Prevention of Osteoporosis in Ovariectomized Rats