J Korean Med Sci.
2004 Feb;19(1):1-7. 10.3346/jkms.2004.19.1.1.
Molecular Motor Proteins of the Kinesin Superfamily Proteins (KIFs): Structure, Cargo and Disease
- Affiliations
-
- 1Department of Microbiology, College of Medicine, Inje University, Busan, Korea. daehyun@ijnc.inje.ac.kr
- 2Department of Psychiatry, College of Medicine, Inje University, Busan, Korea.
- KMID: 1785685
- DOI: http://doi.org/10.3346/jkms.2004.19.1.1
Abstract
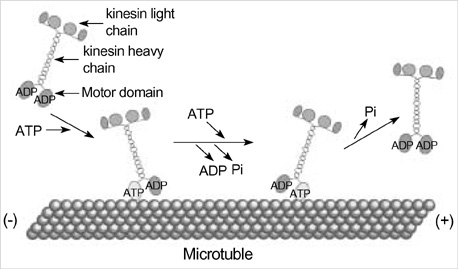
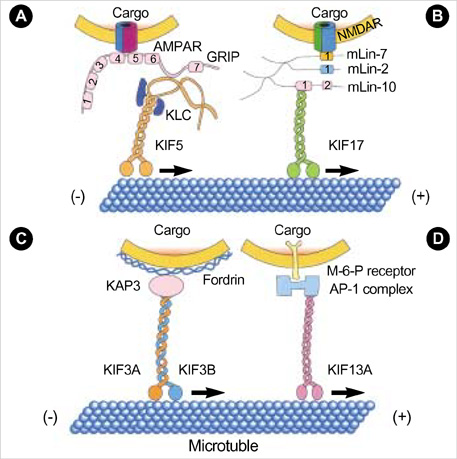
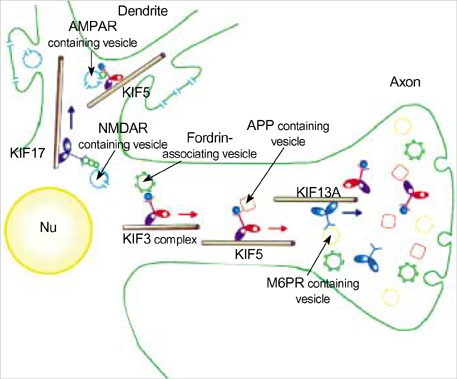
- Intracellular organelle transport is essential for morphogenesis and functioning of the cell. Kinesins and kinesin-related proteins make up a large superfamily of molecular motors that transport cargoes such as vesicles, organelles (e.g. mitochondria, peroxisomes, lysosomes), protein complexes (e.g. elements of the cytoskeleton, virus particles), and mRNAs in a microtubule- and ATP-dependent manner in neuronal and non-neuronal cells. Until now, more than 45 kinesin superfamily proteins (KIFs) have been identified in the mouse and human genomes. Elucidating the transport pathways mediated by kinesins, the identities of the cargoes moved, and the nature of the proteins that link kinesin motors to cargoes are areas of intense investigation. This review focuses on the structure, the binding partners of kinesins and kinesin-based human diseases.
Keyword
MeSH Terms
-
Adenosine Triphosphate/metabolism
Alzheimer Disease/metabolism
Animals
Biological Transport
Cytoplasm/metabolism
Diabetes Mellitus/metabolism
Human
Kinesin/*chemistry/*metabolism
Mice
Microtubule-Associated Proteins/chemistry
Microtubules/metabolism
Models, Biological
Neurons/metabolism
Protein Binding
Support, Non-U.S. Gov't
Figure
Cited by 1 articles
-
Sorting Nexin 17 Interacts Directly with Kinesin Superfamily KIF1Bβ Protein
Dae-Hyun Seog, Jin Han
Korean J Physiol Pharmacol. 2008;12(4):199-204. doi: 10.4196/kjpp.2008.12.4.199.
Reference
-
1. Vale RD, Reese TS, Sheetz MP. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985. 42:39–50.
Article2. Miki H, Setou M, Kaneshiro K, Hirokawa N. All kinesin superfamily protein, KIF, genes in mouse and human. Proc Natl Acad Sci USA. 2001. 98:7004–7011.
Article3. Hirokawa N. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science. 1998. 279:519–526.
Article4. Vale RD, Fletterick RJ. The design plan of kinesin motors. Annu Rev Cell Dev Biol. 1997. 13:745–777.
Article5. Sharp DJ, Rogers GC, Scholey JM. Microtubule motors in mitosis. Nature. 2000. 407:41–47.
Article6. Bloom GS, Wagner MC, Pfister KK, Brady ST. Native structure and physical properties of bovine brain kinesin and identification of the ATP-binding subunit polypeptide. Biochemistry. 1988. 27:3409–3416.
Article7. Yang JT, Laymon RA, Goldstein LS. A three-domain structure of kinesin heavy chain revealed by DNA sequence and microtubule binding analyses. Cell. 1989. 56:879–889.
Article8. Karcher RL, Deacon SW, Gelfand VI. Motor-cargo interactions: the key to transport specificity. Trends Cell Biol. 2002. 12:21–27.
Article9. Seiler S, Kirchner J, Horn C, Kallipolitou A, Woehlke G, Schliwa M. Cargo binding and regulatory sites in the tail of fungal conventional kinesin. Nat Cell Biol. 2000. 2:333–338.
Article10. Dong H, O'Brien RJ, Fung ET, Lanahan AA, Worley PF, Huganir RL. GRIP: a synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997. 386:279–284.
Article11. Srivastava S, Osten P, Vilim FS, Khatri L, Inman G, States B, Daly C, DeSouza S, Abagyan R, Valtschanoff JG, Weinberg RJ, Ziff EB. Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron. 1998. 21:581–591.
Article12. Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M, Hirokawa N. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 2002. 417:83–87.
Article13. Bowman AB, Kamal A, Ritchings BW, Philp AV, McGrail M, Gindhart JG, Goldstein LS. Kinesin-dependent axonal transport is mediated by the sunday driver (SYD) protein. Cell. 2000. 103:583–594.
Article14. Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000. 288:1796–1802.
Article15. Guillaud L, Setou M, Hirokawa N. KIF17 dynamics and regulation of NR2B trafficking in hippocampal neurons. J Neurosci. 2003. 23:131–140.
Article16. Kondo S, Sato-Yoshitake R, Noda Y, Aizawa H, Nakata T, Matsuura Y, Hirokawa N. KIF3A is a new microtubule-based anterograde motor in the nerve axon. J Cell Biol. 1994. 125:1095–1107.
Article17. Yamazaki H, Nakata T, Okada Y, Hirokawa N. KIF3A/B: a heterodimeric kinesin superfamily protein that works as a microtubule plus end-directed motor for membrane organelle transport. J Cell Biol. 1995. 130:1387–1399.
Article18. Takeda S, Yamazaki H, Seog DH, Kanai Y, Terada S, Hirokawa N. Kinesin superfamily protein 3 (KIF3) motor transports fodrin-associating vesicles important for neurite building. J Cell Biol. 2000. 148:1255–1265.
Article19. Kirchhausen T. Clathrin adaptors really adapt. Cell. 2002. 109:413–416.
Article20. Nakagawa T, Setou M, Seog D, Ogasawara K, Dohmae N, Takio K, Hirokawa N. A novel motor, KIF13A, transports mannose-6-phosphate receptor to plasma membrane through direct interaction with AP-1 complex. Cell. 2000. 103:569–581.
Article21. Vale RD. The molecular motor toolbox for intracellular transport. Cell. 2003. 112:467–480.
Article22. Zhao C, Takita J, Tanaka Y, Setou M, Nakagawa T, Takeda S, Yang HW, Terada S, Nakata T, Takei Y, Saito M, Tsuji S, Hayashi Y, Hirokawa N. Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1B beta. Cell. 2001. 105:587–597.23. Harding AE, Thomas PK. Genetic aspects of hereditary motor and sensory neuropathy (types I and II). J Med Genet. 1980. 17:329–336.
Article24. Kim SW, Lee KS, Jin HS, Lee TM, Koo SK, Lee YJ, Jung SC. Rapid detection of duplication/deletion of the PMP22 gene in patients with Charcot-Marie-Tooth disease type 1A and hereditary neuropathy with liability to pressure palsy by real-time quantitative PCR using SYBR Green I Dye. J Korean Med Sci. 2003. 18:727–732.25. Nangaku M, Sato-Yoshitake R, Okada Y, Noda Y, Takemura R, Yamazaki H, Hirokawa N. KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell. 1994. 79:1209–1220.
Article26. Okada Y, Yamazaki H, Sekine-Aizawa Y, Hirokawa N. The neuron-specific kinesin superfamily protein KIF1A is a unique monomeric motor for anterograde axonal transport of synaptic vesicle precursors. Cell. 1995. 81:769–780.27. Yonekawa Y, Harada A, Okada Y, Funakoshi T, Kanai Y, Takei Y, Terada S, Noda T, Hirokawa N. Defect in synaptic vesicle precursor transport and neuronal cell death in KIF1A motor protein-deficient mice. J Cell Biol. 1998. 141:431–441.
Article28. Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002. 13:2384–2398.
Article29. Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA. 2003. 100:5286–5291.
Article30. Wheatley DN. Primary cilia in normal and pathological tissues. Pathobiology. 1995. 63:222–238.
Article31. Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002. 3:813–825.
Article32. Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998. 95:829–837.
Article33. Takeda S, Yonekawa Y, Tanaka Y, Okada Y, Nonaka S, Hirokawa N. Left-right asymmetry and kinesin superfamily protein KIF3A: new insights in determination of laterality and mesoderm induction by kif3A-/- mice analysis. J Cell Biol. 1999. 145:825–836.
Article34. Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci USA. 1999. 96:5043–5048.
Article35. Muresan V, Abramson T, Lyass A, Winter D, Porro E, Hong F, Chamberlin NL, Schnapp BJ. KIF3C and KIF3A form a novel neuronal heteromeric kinesin that associates with membrane vesicles. Mol Biol Cell. 1998. 9:637–652.
Article36. An CH, Choi JC, Lee BH, Park YB, Jee HS, Park SJ, Kim JY, Park IW, Choi BW, Hue SH. One case of Kartagener's syndrome with extracemtral microtubule in cilia. Korean J Med. 2000. 59:230–234.37. Yang Z, Goldstein LS. Characterization of the KIF3C neural kinesin-like motor from mouse. Mol Biol Cell. 1998. 9:249–261.
Article38. Ray K, Perez SE, Yang Z, Xu J, Ritchings BW, Steller H, Goldstein LS. Kinesin-II is required for axonal transport of choline acetyltransferase in Drosophila. J Cell Biol. 1999. 147:507–518.
Article39. Price DL, Sisodia SS. Mutant genes in familial Alzheimer's disease and transgenic models. Annu Rev Neurosci. 1998. 21:479–505.
Article40. Selkoe DJ, Podlisny MB, Joachim CL, Vickers EA, Lee G, Fritz LC, Oltersdorf T. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci USA. 1988. 85:7341–7345.
Article41. Selkoe DJ. The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer's disease. Trends Cell Biol. 1998. 8:447–453.42. Schnapp BJ, Vale RD, Sheetz MP, Reese TS. Single microtubules from squid axoplasm support bidirectional movement of organelles. Cell. 1985. 40:455–462.
Article43. Scholey JM, Heuser J, Yang JT, Goldstein LS. Identification of globular mechanochemical heads of kinesin. Nature. 1989. 338:355–357.
Article44. Blatch GL, Lassle M. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays. 1999. 21:932–939.
Article45. Ferreira A, Niclas J, Vale RD, Banker G, Kosik KS. Suppression of kinesin expression in cultured hippocampal neurons using antisense oligonucleotides. J Cell Biol. 1992. 117:595–606.
Article46. Gunawardena S, Goldstein LS. Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in Drosophila. Neuron. 2001. 32:389–401.
Article47. Kamal A, Almenar-Queralt A, LeBlanc JF, Roberts EA, Goldstein LS. Kinesin-mediated axonal transport of a membrane compartment containing beta-secretase and presenilin-1 requires APP. Nature. 2001. 414:643–648.48. Kamal A, Goldstein LS. Connecting vesicle transport to the cytoskeleton. Curr Opin Cell Biol. 2000. 12:503–508.
Article49. Olson AL, Pessin JE. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu Rev Nutr. 1996. 16:235–256.
Article50. Czech MP, Corvera S. Signaling mechanisms that regulate glucose transport. J Biol Chem. 1999. 274:1865–1868.
Article51. Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002. 3:267–277.
Article52. Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001. 409:729–733.
Article53. Zisman A, Peroni OD, Abel ED, Michael MD, Mauvais-Jarvis F, Lowell BB, Wojtaszewski JF, Hirshman MF, Virkamaki A, Goodyear LJ, Kahn CR, Kahn BB. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med. 2000. 6:924–928.
Article54. Semiz S, Park JG, Nicoloro SM, Furcinitti P, Zhang C, Chawla A, Leszyk J, Czech MP. Conventional kinesin KIF5B mediates insulin-stimulated GLUT4 movements on microtubules. EMBO J. 2003. 22:2387–2399.
Article55. Shin H, Wyszynski M, Huh KH, Valtschanoff JG, Lee JR, Ko J, Streuli M, Weinberg RJ, Sheng M, Kim E. Association of the kinesin motor KIF1A with the multimodular protein liprin-alpha. J Biol Chem. 2003. 278:11393–11401.56. Mok H, Shin H, Kim S, Lee JR, Yoon J, Kim E. Association of the kinesin superfamily motor protein KIF1Balpha with postsynaptic density-95 (PSD-95), synapse-associated protein-97, and synaptic scaffolding molecule PSD-95/discs large/zona occludens-1 proteins. J Neurosci. 2002. 22:5253–5258.57. Dorner C, Ullrich A, Haring HU, Lammers R. The kinesin-like motor protein KIF1C occurs in intact cells as a dimer and associates with proteins of the 14-3-3 family. J Biol Chem. 1999. 274:33654–33660.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Kinesin Superfamily KIF5 Proteins Bind to betaIII Spectrin
- Kinesin Superfamily KIF1Balpha Protein Binds to the PDZ Domain of MALS-3
- Kinesin Superfamily KIF1A Protein Binds to Synaptotagmin XI
- Sorting Nexin 17 Interacts Directly with Kinesin Superfamily KIF1B beta Protein
- Kinesin Spindle Protein Inhibition in Translational Research