Yonsei Med J.
2008 Dec;49(6):1017-1022. 10.3349/ymj.2008.49.6.1017.
Surveillance of Stool Samples for the Presence of Enterobacter sakazakii among Korean People
- Affiliations
-
- 1Division of Health Research and Planning, Gyeonggi-do Research Institute of Health and Environment, Suwon, Korea. chs@catholic.ac.kr
- 2Division of Enteric Bacterial Infections, Center for Infectious Diseases, National Institute of Health, Seoul, Korea.
- 3Department of Laboratory Medicine, The Catholic University College of Medicine, Uijeong-bu, Korea.
- 4Department of Internal Medicine, The Catholic University College of Medicine, Uijeong-bu, Korea.
- KMID: 1782952
- DOI: http://doi.org/10.3349/ymj.2008.49.6.1017
Abstract
- PURPOSE
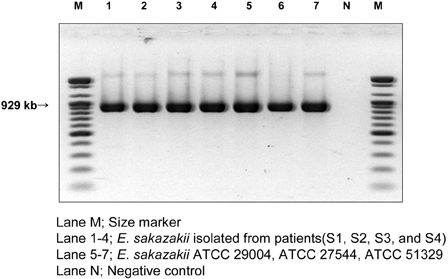
Enterobacter sakazakii (E. sakazakii) infections are an important cause of life-threatening meningitis, septicemia, and necrotizing enterocolitis in infants. Dried infant formula milk is an important vehicle for E. sakazakii infection. E. sakazakii was isolated in Korea from dried infant formula milk. Although E. sakazakii infection of infants may occur in Korea, its prevalence has not yet been documented. Therefore, we determined the prevalence of E. sakazakii and documented symptoms. MATERIALS AND METHODS: Between March and October 2006, 1,146 stool samples were collected from patients at Uijeongbu St. Mary's Hospital. Each fecal swab was dissolved in 10mL of buffered peptone solution, and enriched culture was streaked onto Druggan-Forsythe-Iversen (DFI) agar. Presumptive E. sakazakii colonies that exhibited a blue-green color during culture on DFI medium were selected. The identity of colonies that developed yellow pigment during culture on TSA was determined using the Vitek system and PCR. RESULTS: We isolated 4 E. sakazakii strains whose 16S rRNA sequence alignments had a similarity of 99% with those of 3 E. sakazakii ATCC strains. CONCLUSION: This is the first report on isolation of E. sakazakii from stool samples and to document the symptoms of Korean patients.
Keyword
MeSH Terms
-
Adolescent
Adult
Aged
Base Sequence
Child
Child, Preschool
Drug Resistance, Multiple, Bacterial
*Enterobacter sakazakii/drug effects/genetics/isolation & purification
Enterobacteriaceae Infections/diagnosis/*epidemiology/microbiology
Feces/microbiology
Female
Humans
Infant
Infant, Newborn
Korea/epidemiology
Male
Middle Aged
Molecular Sequence Data
RNA, Bacterial/genetics
RNA, Ribosomal, 16S/genetics
Sequence Homology, Nucleic Acid
Young Adult
Figure
Reference
-
1. Farmer JJ III, Asbury MA, Hickman FW, Brenner DJ. Enterobacteriaceae Study Group. Enterobacter sakazakii: a new species of "Enterobacteriaceae" isolated from clinical specimens. Int J Syst Bacteriol. 1980. 30:569–584.
Article2. Lai KK. Enterobacter sakazakii infections among neonates, infants, children, and adults. Case reports and a review of the literature. Medicine (Baltimore). 2001. 80:113–122.
Article3. Nazarowec-White M, Farber JM. Enterobacter sakazakii: a review. Int J Food Microbiol. 1997. 34:103–113.4. Hawkins RE, Lissner CR, Sanford JP. Enterobacter sakazakii bacteremia in an adult. South Med J. 1991. 84:793–795.5. Ongrádi J. Vaginal infection by Enterobacter sakazakii. Sex Transm Infect. 2002. 78:467.6. Centers for Disease Control and Prevention (CDC). Enterobacter sakazakii infections associated with the use of powdered infant formula-Tennessee, 2001. MMWR Morb Mortal Wkly Rep. 2002. 51:297–300.7. Block C, Peleg O, Minster N, Bar-Oz B, Simhon A, Arad I, et al. Cluster of neonatal infections in Jerusalem due to unusual biochemical variant of Enterobacter sakazakii. Eur J Clin Microbiol Infect Dis. 2002. 21:613–616.
Article8. Biering G, Karlsson S, Clark NC, Jónsdóttir KE, Lúdvígsson P, Steingrímsson O. Three cases of neonatal meningitis caused by Enterobacter sakazakii in powdered milk. J Clin Microbiol. 1989. 27:2054–2056.
Article9. Drudy D, Mullane NR, Quinn T, Wall PG, Fanning S. Enterobacter sakazakii: an emerging pathogen in powdered infant formula. Clin Infect Dis. 2006. 42:996–1002.10. Iversen C, Forsythe S. Risk profile of Enterobacter sakazakii, an emergent pathogen associated with infant milk formula. Trends Food Sci Technol. 2003. 14:443–454.
Article11. Leclercq A, Wanegue C, Baylac P. Comparison of fecal coliform agar and violet red bile lactose agar for fecal coliform enumeration in foods. Appl Environ Microbiol. 2002. 68:1631–1638.
Article12. Skladal P, Mascini M, Salvadori C, Zannoni G. Detection of bacterial contamination in sterile UHT milk using an L-lactate biosensor. Enzyme Microb Technol. 1993. 15:508–512.
Article13. Simmons BP, Gelfand MS, Haas M, Metts L, Ferguson J. Enterobacter sakazakii infections in neonates associated with intrinsic contamination of a powdered infant formula. Infect Control Hosp Epidemiol. 1989. 10:398–401.
Article14. Questions and answers on Enterobacter sakazakii in powdered infant formula. Version 4. 2004 Feb 13. World Health Organization; Available from: http://www.who.int/foodsafety/publications/micro/en/qa2.pdf.15. Yoo MK, Kim SS, Oh SS. Isolation and genotyping of Enterobacter sakazakii from powdered infant formula manufactured in Korea. Food Sci Biotechnol. 2005. 14:875–877.16. van Acker J, de Smet F, Muyldermans G, Bougatef A, Naessens A, Lauwers S. Outbreak of necrotizing enterocolitis associated with Enterobacter sakazakii in powdered milk formula. J Clin Microbiol. 2001. 39:293–297.
Article17. Lehner A, Nitzsche S, Breeuwer P, Diep B, Thelen K, Stephan R. Comparison of two chromogenic media and evaluation of two molecular based identification systems for Enterobacter sakazakii detection. BMC Microbiol. 2006. 6:15.18. Stock I, Wiedemann B. Natural antibiotic susceptibility of Enterobacter amnigenus, Enterobacter cancerogenus, Enterobacter gergoviae and Enterobacter sakazakii strains. Clin Microbiol Infect. 2002. 8:564–578.
Article19. Lehner A, Tasara T, Stephan R. 16S rRNA gene based analysis of Enterobacter sakazakii strains from different sources and development of a PCR assay for identification. BMC Microbiol. 2004. 4:43.20. Muytjens HL, van der Ros-van de Repe J. Comparative in vitro susceptibilities of eight Enterobacter species, with special reference to Enterobacter sakazakii. Antimicrob Agents Chemother. 1986. 29:367–370.
Article21. Iversen C, Waddington M, On SL, Forsythe S. Identification and phylogeny of Enterobacter sakazakii relative to Enterobacter and Citrobacter species. J Clin Microbiol. 2004. 42:5368–5370.
Article22. Seo KH, Brackett RE. Rapid, specific detection of Enterobacter sakazakii in infant formula using a real-time PCR assay. J Food Prot. 2005. 68:59–63.
Article23. Malorny B, Wagner M. Detection of Enterobacter sakazakii strains by real-time PCR. J Food Prot. 2005. 68:1623–1627.24. Kolbert CP, Persing DH. Ribosomal DNA sequencing as a tool for identification of bacterial pathogens. Curr Opin Microbiol. 1999. 2:299–305.
Article25. Woo PC, Ng KH, Lau SK, Yip KT, Fung AM, Leung KW, et al. Usefulness of the MicroSeq 500 16S ribosomal DNA-based bacterial identification system for identification of clinically significant bacterial isolates with ambiguous biochemical profiles. J Clin Microbiol. 2003. 41:1996–2001.
Article26. Hassan AA, Akineden O, Kress C, Estuningsih S, Schneider E, Usleber E. Characterization of the gene encoding the 16S rRNA of Enterobacter sakazakii and development of a species-specific PCR method. Int J Food Microbiol. 2007. 116:214–220.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Enterobacter sakazakii Epidural Abscess in Neonate
- Cronobacter sakazakii Infection Induced Fatal Clinical Sequels Including Meningitis in Neonatal ICR Mice
- Evaluation of commercial probiotic lactic cultures against biofilm formation by Cronobacter sakazakii
- Activity of cefepime against enterobacter cloacae, serratin marcesc- ens, pseudomonas aeruginosa and other aerobic gram-negative bacilli
- Intraventricular Pefloxacine Therapy for a Cerebral Ventriculitis by Enterobacter Aerogenes: Case Report