J Korean Med Sci.
2006 Oct;21(5):877-882. 10.3346/jkms.2006.21.5.877.
Overexpression of CIITA in T Cells Aggravates Th2-Mediated Colitis in Mice
- Affiliations
-
- 1Department of Pathology, Hallym University College of Medicine, Chuncheon, Korea.
- 2Department of Pathology, Seoul National University College of Medicine, Seoul, Korea. jungkc66@snu.ac.kr
- 3Graduate Program of Immunology, Seoul National University College of Medicine, Seoul, Korea.
- 4Center for Animal Resource Development, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1781909
- DOI: http://doi.org/10.3346/jkms.2006.21.5.877
Abstract
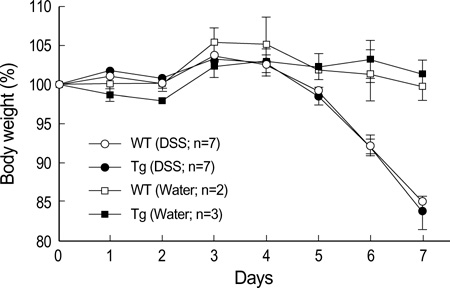
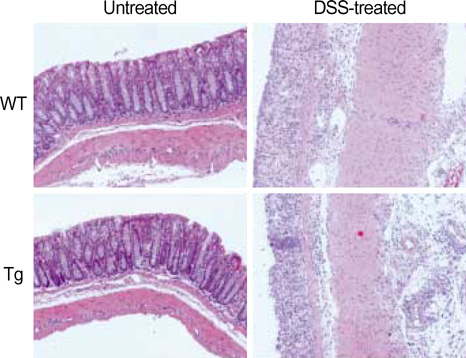
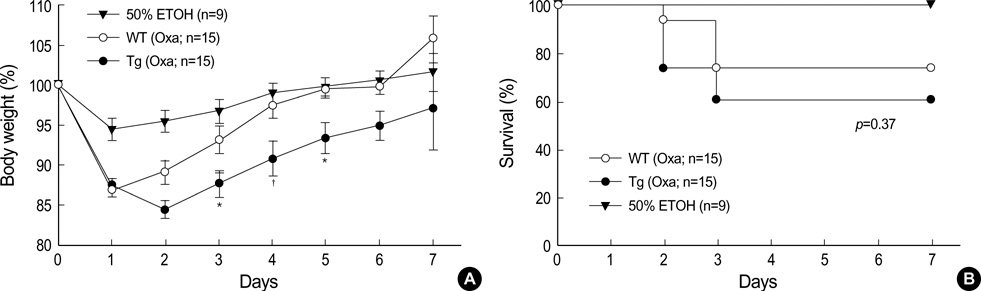
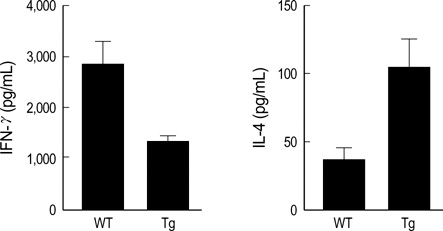
- The MHC class II transactivator (CIITA) is the master transcriptional regulator of genes involved in MHC class II restricted antigen presentation. Previously we suggested another role of CIITA in Th1/Th2 balance by demonstrating that forced expression of CIITA in murine T cells repressed Th1 immunity both in vitro and in vivo. However, the results were contradictory to the report that CIITA functioned to suppress the production of Th2 cytokine by CD4+T cells in CIITA deficient mice. In this study, we investigated the influence of constitutive expression of CIITA in T cells on Th2 immune response in vivo using murine experimental colitis model. In the dextran sodium sulfate-induced acute colitis, a disease involving innate immunity, CIITA transgenic mice and wild type control mice showed similar progression of the disease. However, the development of oxazolone-induced colitis, a colitis mediated by predominantly Th2 immune response, was aggravated in CIITA-transgenic mice. And, CD4+T cells from the mesenteric lymph node of CIITA-transgenic mice treated with oxazolone exhibited a high level of IL-4 secretion. Together, these data demonstrate that constitutive expression of CIITA in T cells skews immune response to Th2, resulting in aggravation of Th2-mediated colitis in vivo.
Keyword
MeSH Terms
Figure
Reference
-
1. Chang CH, Fontes JD, Peterlin M, Flavell RA. Class II transactivator (CIITA) is sufficient for the inducible expression of major histocompatibility complex class II genes. J Exp Med. 1994. 180:1367–1374.
Article2. Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-γ mediated by the transactivator gene CIITA. Science. 1994. 265:106–109.3. Chang CH, Flavell RA. Class II transactivator regulates the expression of multiple genes involved in antigen presentation. J Exp Med. 1995. 181:765–767.
Article4. Dong Y, Rohn WM, Benveniste EN. IFN-γ regulation of the type IV class II transactivator promoter in astrocytes. J Immunol. 1999. 162:4731–4739.5. Ting JP, Trowsdale J. Genetic control of MHC class II expression. Cell. 2002. 109:Suppl. S21–S33.
Article6. Xu Y, Wang L, Buttice G, Sengupta PK, Smith BD. Major histocompatibility class II transactivator (CIITA) mediates repression of collagen (COL1A2) transcription by interferonγ (IFN-γ). J Biol Chem. 2004. 279:41319–41332.7. Nozell S, Ma Z, Wilson C, Shah R, Benveniste EN. Class II major histocompatibility complex transactivator (CIITA) inhibits matrix metalloproteinase-9 gene expression. J Biol Chem. 2004. 279:38577–38589.
Article8. Sisk TJ, Gourley T, Roys S, Chang CH. MHC class II transactivator inhibits IL-4 gene transcription by competing with NF-AT to bind the coactivator CREB binding protein (CBP)/p300. J Immunol. 2000. 165:2511–2517.
Article9. Gourley TS, Chang CH. Cutting edge: the class II transactivator prevents activation-induced cell death by inhibiting fas ligand gene expression. J Immunol. 2001. 166:2917–2921.
Article10. Montani V, Shong M, Taniguchi SI, Suzuki K, Giuliani C, Napolitano G, Saito J, Saji M, Fiorentino B, Reimold AM, Singer DS, Kohn LD. Regulation of major histocompatibility class II gene expression in FRTL-5 thyrocytes: opposite effects of interferon and methimazole. Endocrinology. 1998. 139:290–302.
Article11. Kim WB, Kim TY, Park YJ, Koh JJ, Park DJ, Cho BY. Effect of class 2 transactivator on expression of thyroid transcription factor-1 in FRTL-5 cells. J Korean Soc Endocrinol. 2002. 17:48–56.12. Gourley T, Roys S, Lukacs NW, Kunkel SL, Flavell RA, Chang CH. A novel role for the major histocompatibility complex class II transactivator CIITA in the repression of IL-4 production. Immunity. 1999. 10:377–386.
Article13. Park WS, Bae Y, Chung DH, Choi YL, Kim BK, Sung YC, Choi EY, Park SH, Jung KC. T cell expression of CIITA represses Th1 immunity. Int Immunol. 2004. 16:1355–1364.
Article14. Otten LA, Tacchini-Cottier F, Lohoff M, Annunziato F, Cosmi L, Scarpellino L, Louis J, Steimle V, Reith W, Acha-Orbea H. Deregulated MHC class II transactivator expression leads to a strong Th2 bias in CD4+ T lymphocytes. J Immunol. 2003. 170:1150–1157.15. Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone colitis: A murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998. 188:1929–1939.
Article16. Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002. 17:629–638.
Article17. Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990. 98:694–702.
Article18. Gourley TS, Patel DR, Nickerson K, Hong SC, Chang CH. Aberrant expression of fas ligand in mice deficient for the MHC class II transactivator. J Immunol. 2002. 168:4414–4419.
Article19. Chensue SW, Warmington K, Ruth J, Lincoln P, Kuo MC, Kunkel SL. Cytokine responses during mycobacterial and schistosomal antigen-induced pulmonary granuloma formation. Production of Th1 and Th2 cytokines and relative contribution of tumor necrosis factor. Am J Pathol. 1994. 145:1105–1113.20. Hibi T, Ogata H, Sakuraba A. Animal models of inflammatory bowel disease. J Gastroenterol. 2002. 37:409–417.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long Noncoding RNA FBXL19-AS1-Mediated Ulcerative Colitis-Associated Intestinal Epithelial Barrier Defect
- Sepsis Mortality in CIITA Deficient Mice is Associated with Excessive Release of High-mobility Group Box 1
- Short-Term High Expression of Interferon-Alpha Modulates Progression of Type 1 Diabetes in NOD Mice
- Novel role of MHC class II transactivator in hepatitis B virus replication and viral counteraction
- Pathophysiology of ulcerative colitis - Relationship with genetics and immunity