Korean J Lab Med.
2007 Apr;27(2):151-155. 10.3343/kjlm.2007.27.2.151.
Standardization of Terminology in Laboratory Medicine I
- Affiliations
-
- 1Department of Laboratory Medicine, School of Medicine, Korea University, Seoul, Korea. kaplee@kumc.or.kr
- 2Department of Laboratory Medicine, Seoul National University, Seoul, Korea.
- 3Department of Laboratory Medicine, Ulsan University, Seoul, Korea.
- 4Department of Laboratory Medicine, Kwandong University, Gangneung, Korea.
- 5Department of Laboratory Medicine, Dong-Kook University, Gyeongju, Korea.
- KMID: 1781476
- DOI: http://doi.org/10.3343/kjlm.2007.27.2.151
Abstract
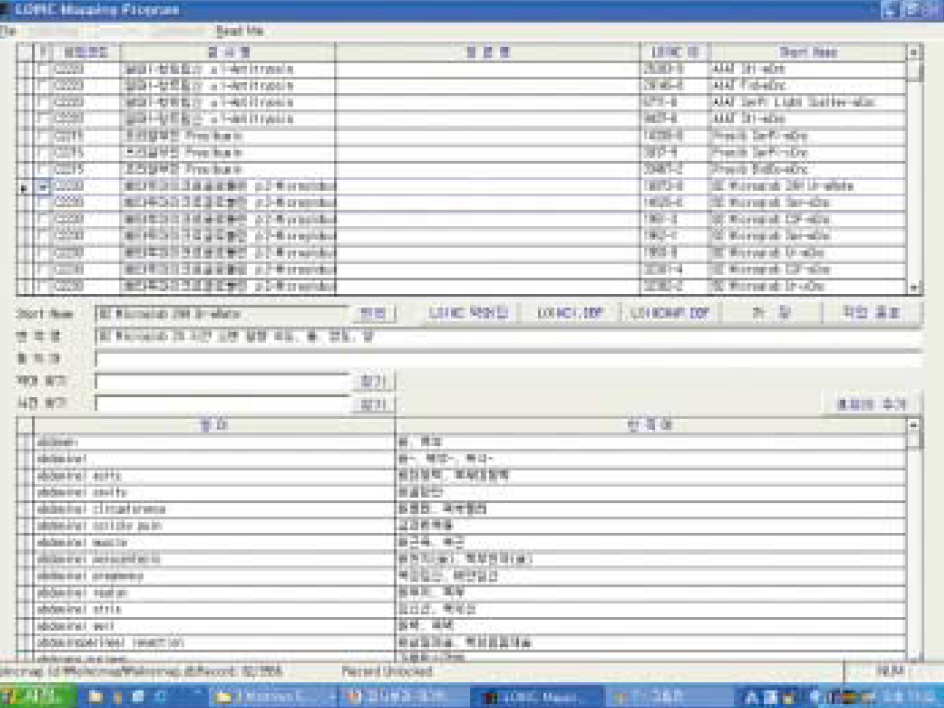
- BACKGROUND: Standardization of medical terminology is essential for data transmission between health-care institutions or clinical laboratories and for maximizing the benefits of information technology. Purpose of our study was to standardize the medical terms used in the clinical laboratory, such as test names, units, terms used in result descriptions, etc. During the first year of the study, we developed a standard database of concept names for laboratory terms, which covered the terms used in government health care centers, their branch offices, and primary health care units. METHODS: Laboratory terms were collected from the electronic data interchange (EDI) codes from National Health Insurance Corporation (NHIC), Logical Observation Identifier Names and Codes (LOINC) database, community health centers and their branch offices, and clinical laboratories of representative university medical centers. For standard expression, we referred to the English-Korean/ Korean-English medical dictionary of Korean Medical Association and the rules for foreign language translation. Programs for mapping between LOINC DB and EDI code and for translating English to Korean were developed. RESULTS: A Korean standard laboratory terminology database containing six axial concept names such as components, property, time aspect, system (specimen), scale type, and method type was established for 7,508 test observations. Short names and a mapping table for EDI codes and Unified Medical Language System (UMLS) were added. Synonym tables for concept names, words used in the database, and six axial terms were prepared to make it easier to find the standard terminology with common terms used in the field of laboratory medicine. CONCLUSIONS: Here we report for the first time a Korean standard laboratory terminology database for test names, result description terms, result units covering most laboratory tests in primary healthcare centers.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Standardization of Terminology in Laboratory Medicine II
Kap No Lee, Jong-Hyun Yoon, Won Ki Min, Hwan Sub Lim, Junghan Song, Seok Lae Chae, Seongsoo Jang, Chang-Seok Ki, Sook Young Bae, Jang Su Kim, Jung-Ah Kwon, Chang Kyu Lee, Soo-Young Yoon
J Korean Med Sci. 2008;23(4):711-713. doi: 10.3346/jkms.2008.23.4.711.Application Guidelines of Logical Observation Identifiers Names and Codes (LOINC) for General Chemistry and Hematology Tests
Kuenyoul Park, Hyejin Ryu, Min-Sun Kim, Ye-Jin Oh, Shinae Yu, Eun-Jung Cho, Sollip Kim, Jae-Woo Chung, Yeo-Min Yun, Young Kyung Lee, Sail Chun
Lab Med Online. 2025;15(1):28-35. doi: 10.47429/lmo.2025.15.1.28.
Reference
-
References
1. Korean Medical Association. English-Korean, Korean-English medical terminology. 4th ed.Korea: Korean Medical Association;2001.2. Forrey AW, McDonald CJ, DeMoor G, Huff SM, Leavelle D, Leland D, et al. Logical observation identifier names and codes (LOINC) database: a public use set of codes and names for electronic reporting of clinical laboratory test results. Clin Chem. 1996; 42:81–90.
Article3. The Regenstrief Institute. LOINC database. 2.13 ed. http://www.regenstrief.org/loinc/. (Updated on Dec.2004.4. McDonald CJ, Huff SM, Suico J, Mercer K. (eds.).LOINC user's guide. http://www.regenstrief.org/loinc/. (updated on Dec.2004.5. National Health Information Standard Committee. National Health Information Standard Committee. http://www.medistds.or.kr/. (Updated on Nov.2006.6. Sakurabayashi I. Coding for clinical laboratory information. Jpn J Clin Pathol. 1997; 45:573–6.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The standardization of the Korean-English veterinary medical terminology
- Toward High-Quality Real-World Laboratory Data in the Era of Healthcare Big Data
- Comparison on the Embryological Terms between the Korean Veterinary Anatomical Terminology (2008) and the Korean Anatomical Terminology (1996)/the Korean Medical Association: Medical Terminology (2009)
- Brief History of Standardization of Korean Terminology in the Field of Pathology
- A Systematic Review of Audiology Terminology